SATURDAY, 01 OCTOBER 2016 / PUBLISHED IN BLOG
Researchers from the Medical University of South Carolina (MUSC) and the University of Pennsylvania have discovered a new methodology for purifying liver cells generated from induced pluripotent stem cells (iPSCs) that could facilitate progress toward an important clinical goal: treating patients with disease-causing liver mutations by transplanting unmutated liver cells derived from their own stem cells.
Background on Liver Cell Generation from iPSCs
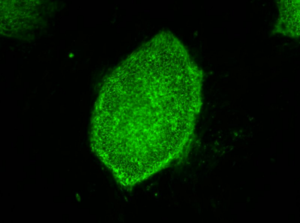
Credit: Image courtesy of Stephen A. Duncan, Ph.D., at the Medical University of South Carolina
Previous attempts to generate liver-like cells from stem cells have yielded heterogeneous cell populations with little similarity to diseased livers in patients.
The Role of the Next Generation Genetic Association Studies Program
The National Heart, Lung, and Blood Institute (NHLBI)’s Next Generation Genetic Association Studies Program (Next Gen) was created to bank stem cell lines sourced from patients in genome-wide association studies (GWAS). The goal of the Next Gen Lipid Conditions sub-section, a collaborative effort between Stephen A. Duncan, Ph.D., chair of regenerative medicine at MUSC, and Daniel J. Rader, M.D., and Edward E. Morrisey, Ph.D., both at the University of Pennsylvania, is to help determine the genetic sources of heart, lung, or blood conditions that also include the liver.
Genome-Wide Association Studies (GWAS)
GWAS studies map the genomes in hundreds of people to look for genetic mutation patterns that differ from the genomes of healthy individuals. As GWAS studies map more genomes, they become more likely to find the correct genetic mutations that cause a disease. Once a panel of suspected mutations is built, stem cells from these individuals can be manipulated in culture dishes to differentiate into any of the body’s cells. The cells can be screened to learn more about the mutations and to test panels of drugs that might ultimately help treat patients harboring a disease.
Challenges in Cell Manipulation Process
Problems arise during the cell manipulation process. For example, iPSCs persistently refuse to mature uniformly into liver-like cells when fed growth factors. Traditionally, antibodies have been used to recognize features of maturity on the surfaces of cells and purify cells that are similar, an approach that has been crucial to stem cell research. But available antibodies that recognize mature liver cells are scanty and tend to recognize many different kinds of cells. The many types of cells in mixed populations have diverse characteristics that can obscure underlying disease-causing genetic variations, which tend to be subtle.
Introduction of Chemo Proteomic Cell Surface Capture (CSC) Technology
Instead of relying on antibodies, Duncan and his team embraced a new technology called chemo proteomic cell surface capture (CSC) technology. CSC technology allowed the researchers to map the most highly produced proteins on the surface of liver cells during the final stages of differentiation of stem cells into liver cells. The most abundant protein was targeted with an antibody labeled with a fluorescent marker and used to sort the mature liver cells from the rest.
Successful Generation of Pure Liver-like Cells
The procedure was highly successful: The team had a population of highly pure, homogeneous, and mature liver-like cells. Labeled cells had far more similar traits of mature hepatocytes than unlabeled cells. Pluripotent stem cells that had not differentiated were excluded from the group of labeled cells.
“That’s important,” says Duncan. “If you’re wanting to transplant cells into somebody that has liver disease, you really don’t want to be transplanting pluripotent cells because pluripotent cells form tumors called teratocarcinomas.”
Future Implications
Duncan cautioned that transplantation of iPSC-derived liver cells is not yet ready for translation to the clinic, but the technology for sorting homogeneous liver cells can be used now to successfully and accurately model and study disease in the cell culture dish.
“We think that the ability to generate pure populations will get rid of the variability, and therefore really help us combine with GWAS studies to identify allelic variations that are causative of a disease, at least in the liver,” he says.
Contributions to the Study
Researchers at the University of Minnesota (Minneapolis) and the Medical College of Wisconsin (Milwaukee) contributed to the study, published August 25, in Stem Cell Reports.