Stem Cell Treatments Normally Used for Cancer Patients are Helping Multiple Sclerosis Patients
MONDAY, 04 APRIL 2016 / PUBLISHED IN BLOG
Introduction
The British Broadcasting Corporation (BBC) recently reported that stem cell transplant treatments normally used for cancer patients are helping Multiple Sclerosis (MS) patients in the UK. According to the January 18, 2016 report, 20 patients received bone marrow stem cell transplants using their own stem cells, and at least some of the patients who were paralyzed by MS are able to walk again post-treatment.
Impact of Multiple Sclerosis in the UK
Approximately 100,000 people in the United Kingdom suffer from MS, with most new patients diagnosed between the ages of 20 and 30 years. “To have a treatment which can potentially reverse disability is really a major achievement,” says Prof Basil Sharrack of Sheffield’s Royal Hallamshire Hospital in Sheffield, England.
Autologous Hematopoietic Stem Cell Transplantation (HSCT)
The treatment, known as autologous hematopoietic stem cell transplantation (HSCT), involves the intravenous infusion of autologous or allogeneic stem cells harvested from the patient’s own bone marrow to reestablish hematopoietic function (formation of blood or blood cells) in patients whose bone marrow or immune system is damaged or defective by chemotherapy. Using stem cells harvested from the patient’s bone marrow helps rebuild the immune system. The theory is that these newly harvested cells are at such an early stage in development that the cellular defects that result in MS do not exist. “The immune system is being reset or rebooted back to a time point before it caused MS,” says Prof John Snowden, consultant hematologist at Royal Hallamshire Hospital.
Patient Success Stories
The BBC’s Panorama program spoke to several MS patients who have undergone the stem cell transplant. Steven Storey was diagnosed with MS in 2013 and, within a year, went from being an able-bodied athlete to wheelchair dependent and losing sensation in much of his body. “I went from running marathons to needing 24-hour acute care. At one point I couldn’t even hold a spoon and feed myself,” Storey says.

Clinical Trials and Research
The Royal Hallamshire Hospital along with hospitals in the United States, Sweden, and Brazil, is part of an international clinical trial called MIST that is assessing the long-term benefits of the stem cell procedure on MS patients. Study participants all have relapsing-remitting MS (RRMS) and received intensive chemotherapy to completely destroy the patients’ immune systems.
Cost and Accessibility of Treatment
Treatment costs are about the same as the annual cost for existing treatments, and the stem cell treatment does not require the use of new or existing medications. Prof Richard Burt of Northwestern University in Chicago carried out the first hematopoietic stem cell transplantation for MS in 1995, and is coordinating this current MIST international trial, which began in 2006. “There has been resistance to this in the pharma and academic world,” Burt says. “This is not a technology you can patent and we have achieved this without industry backing.”
Study Results and Future Implications
A study published last year involving MS patients in Chicago showed significant reductions in neurological disability, and for some, the improvements persisted for at least four years, although there was no comparative control group. The outcomes of the current international trial will be reported in 2018 and may determine whether the stem cell transplant becomes a standard in the United Kingdom’s healthcare system for many MS patients. “Ongoing research suggests stem cell treatments such as HSCT could offer hope, and it’s clear that in the cases highlighted by Panorama they’ve had a life-changing impact,” says Emma Gray, M.D., head of clinical trials at UK’s MS Society.
- Published in Blog
How Stem Cell Therapies Can Help Heal Sports Injuries
MONDAY, 14 MARCH 2016 / PUBLISHED IN BLOG
Introduction to Stem Cell Therapies in Sports Medicine
Continuing our recent discussion of stem cell therapies for sports injuries, the use of mesenchymal stem cells (MSCs) in orthopedic medicine can help in the repair of damaged tissue by harnessing the healing power of undifferentiated cells that form all other cells in our bodies. The process involves isolating these stem cells from a sample of your blood, bone marrow, or adipose tissue (fat cells), and injecting it into the damaged body part to promote healing. Platelet-rich plasma (PRP), a concentrated suspension of platelets (blood cells that cause clotting of blood) and growth factors, is also used to assist the process of repair.
Cartilage Damage

Cartilage has long been considered an ideal candidate for cell therapy as it is a relatively simple tissue, composed of one cell type, chondrocytes, and does not have a substantial blood supply network. Of particular interest to researchers is the repair of cartilage tissue in the knee, also called the meniscus of the knee. The meniscus is responsible for distributing the body’s weight at the knee joint when there is movement between the upper and lower leg. Only one third of meniscus cartilage has a blood supply, and as the blood supply allows healing factors and stem cells attached to the blood vessels (called perivascular stem cells) to access the damaged site, the meniscus’s natural lack of blood supply impairs healing of this tissue. Damage to this tissue is common in athletes, and is the target for surgery in 60 percent of patients undergoing knee operations, which usually involves the partial or complete removal of the meniscus, which can lead to long-term cartilage degeneration and osteoarthritis.
Recently, researchers have increased their focus on the use of MSCs for treatment of cartilage damage in the knee. Some data from animal models suggest that damaged cartilage undergoes healing more efficiently when MSCs are injected into the injury, and this can be further enhanced if the MSCs are modified to produce growth factors associated with cartilage. It has been shown that once the MSCs are injected into the knee they attach themselves to the site of damage and begin to change into chondrocytes, promoting healing and repair. A small number of completed clinical trials in humans using MSCs to treat cartilage damage have reported some encouraging results, but these studies used very few patients, making it difficult to accurately interpret the results. There are currently a number of ongoing trials using larger groups of patients, and the hope is that these will provide more definite information about the role MSCs play in cartilage repair.
Tendinopathy

Tendinopathy relates to injuries that affect tendons – the long fibrous tissues that connect and transmit force from muscles to bones. Tendons become strained and damaged through repetitive use, making tendinopathy a common injury among athletes. Tendinopathy has been linked to 30 percent of all running-related injuries, and up to 40 percent of tennis players suffer from some form of elbow tendinopathy or “tennis elbow.” Damage occurs to the collagen fibers that make up the tendon, and this damage is repaired by the body through a process of inflammation and production of new fibers that fuse together with the undamaged tissue. However, this natural healing process can take up to a year to resolve, and results in the formation of a scar on the tendon tissue, reducing the tendon’s natural elasticity, decreasing the amount of energy the tissue can store and resulting in a weakening of tendon.
MSCs have the ability to generate cells called tenoblasts that mature into tenocytes. These tenocytes are responsible for producing collagen in tendons. This link between MSCs and collagen is the focus for researchers investigating how stem cells may help treat tendinopathy. Substantial research has been carried out on racehorses as they suffer from high rates of tendinopathy, and the injury is similar to that found in humans. Researchers discovered that by injecting MSCs isolated from an injured horse’s own bone marrow into the damaged tendon, recurrence rates were almost cut in half compared to horses that receive traditional medical management for this type of injury. A later study by the same group showed the MSCs improved repair, resulting in reduced stiffness of the tissue, decreased scarring, and better fusion of the new fibers with the existing, undamaged tendon. It is not yet clear if these results are due to MSCs producing new tenocytes or their ability to modulate the environment around the tendinopathy, as described above. These promising results paved the way for the first pilot study in humans.
Bone Repair

Bones are unique in that they have the ability to regenerate throughout life. Upon injury, such as a fracture, a series of events occur to initiate healing of the damaged bone. Initially, there is inflammation at the site of injury, and a large number of signals are sent out. These signals attract MSCs, which begin to divide and increase their numbers. The MSCs then change into either chondrocytes, the cells responsible for making a type of cartilage scaffold, or osteoblasts, the cells that deposit the proteins and minerals that comprise the hard bone onto the cartilage. Finally, these new structures are altered to restore shape and function to the repaired bone. A number of studies carried out in animals have demonstrated that direct injection or infusing the blood with MSCs can help heal fractures that previously failed to heal naturally. However, as was the case with tendinopathy, it is not yet clear if these external MSCs work by generating more bone-producing cells or through their ability to reduce inflammation and encourage restoration of the blood supply to injured bone, or both.
Brain Injury in Sports

There is mounting evidence that those taking part in sports where they are exposed to repetitive trauma to the head and brain are at a higher risk of developing neurodegenerative disorders, some of which are targets for stem cell treatments. For example, it has been reported that the rate of these diseases, like Alzheimer’s Disease, were almost four times higher in professional American football players compared to the general population. While the cause of this disease is not yet clear, it is associated with abnormal accumulation of proteins in neural cells that eventually undergo cell death and patients develop dementia. Researchers have attempted a number of strategies to investigate treatments of this disease in mice, including introducing neural stem cells that could produce healthy neurons. While some of these experiments have demonstrated positive, if limited, effects, to date there are no stem cell treatments available for Alzheimer’s Disease.
Boxers suffering from dementia pugilistica, a disease thought to result from damage to nerve cells, can also demonstrate some symptoms of Parkinson’s Disease (among others). In healthy brains, specialized nerve cells called dopaminergic neurons produce dopamine, a chemical that transmits signals to the part of the brain responsible for movement. The characteristic tremor and rigidity associated with Parkinson’s Disease is due to the loss of these dopaminergic neurons and the resulting loss of dopamine production. Researchers are able to use stem cells to generate dopaminergic neurons in the lab that are used to study the development and pathology of this disease. While a recent study reported that dopaminergic neurons derived from human embryonic stem cells improved some symptoms of the disease in mice and rats, stem cell-based treatments are still in the development phase.
- Published in Blog
What’s the Skinny on Adipose Stem Cells Derived From Fat?
WEDNESDAY, 13 JANUARY 2016 / PUBLISHED IN BLOG
Introduction to Adipose Stem Cells
In this blog, I’ll share some of the results we’ve had using stem cell therapies in different ways to show you how you can utilize them in your office or clinic. Let’s start with stem cell treatments for cosmetic regenerative tissue enhancement. The procedure starts with taking fat from one location on the patient’s body and relocating it to the area you’re trying to enhance and combining that fat with a population of adipose (fat-derived) stem cells for best results.
The Science Behind Adipose Stem Cells
This theory, in part, was first published back in 2006 by Kotaro Yoshimura, M.D., Associate Professor, Department of Plastic Surgery at the University of Tokyo. Dr. Yoshimura demonstrated that stem cells harvested from fat are actually responsible for creating new adipocytes.
Does this mean fat is our friend? When it comes to therapeutic tissue treatments, it sure is! We used to believe that we had a set number of adipocytes and that these either grew or shrank depending on the amount of fat that our bodies were gaining or holding, but we now know better. Everyone has a population of stem cells that exist within their fat tissue that is responsible for replacing or replenishing mature adipocytes, and they’ll grow with weight gain. By attaching to fat tissue, those stem cells will actually help support expansion or weight gain.
Breast Augmentation and Skin Rejuvenation

Take, for instance, breast augmentation using this process. By taking fat from one location, relocating it, and adding stem cells with the fat to the breast tissue, you can reduce reabsorption of the fat tissue. In addition to being able to perform fat transfer for breast augmentation, you can also utilize the stem cells and platelet-rich plasma (PRP) when you need to rejuvenate the skin as well. One example would be a patient who had received an injection of stem cells plus platelet-rich plasma without a fat graft: in this case, the cells will be very angiogenic in nature, creating new blood vessels and generating a youthful glow. The cells can also help with collagen production so the patient gets smoother skin and help with scarring or the appearance of unevenness on the skin.
Adipose Stem Cells in Orthopedics
Adipose stem cells can also be utilized for regenerative results in orthopedics. A typical technique is to isolate the platelet-rich plasma from the peripheral blood and combine it with stem cells from the fat tissue. Our preference is to utilize the adipose stem cells, again, because of the massive volume of stem cells fat tissue delivers. We can obtain about five hundred times more mesenchymal type stem cells—stem cells that can differentiate into a variety of cell types—from adipose tissue than we can obtain from bone marrow. For this reason, in most cases we utilize the cells from the adipose tissue rather than the bone marrow.
Protocols and Success Stories in Orthopedics
This protocol comes courtesy of Joseph Purita, M.D., a member of the Global Stem Cells Group Advisory Board and a pioneer in the use of stem cells and PRP therapy for orthopedic surgery. Dr. Purita’s protocol is to inject the adipose cells plus the PRP intra-articularly to the affected joint.
This therapy has also been used successfully in animals. For instance, in the case of a horse with a chronic, non-healing tear in the ligament considered so chronic that they were going to put it down, an injection of the platelet-rich plasma plus the adipose stem cells directly into the lesion resulted in a complete resolution of the non-healing ligament within six months post-treatment.
Case Studies: Human Applications

Again, courtesy of Dr. Purita, is another example of a patient with avascular necrosis who had been told that she needed a total knee replacement. She was getting her knee drained once per week, had severe swelling and pain, and was not able to perform, pretty much, any activities due to her joint pain. After injection of the adipose cells plus the PRP, the patient was essentially pain-free, she was able to play tennis weekly, and there was complete resolution of the avascular necrosis, according to MRIs six months post-treatment.
Another example is a patient who was hit by a bus and thrown into a house, resulting in a non-union bone fracture that never healed properly. In this case, the patient was treated at the Hospital Angeles in collaboration with the Regenerative Medicine Institute. The patient had not been able to bear weight on the leg for more than two years. After an injection of stem cells plus a bone matrix, at the three-month follow-up, there was full continuity down the length of the bone, and for the first time in more than two years, the patient was able to bear weight.
Conclusion: The Future of Adipose Stem Cells
Treatments using adipose (fat)-derived stem cells, in combination with PRP and other regenerative medicine therapies, are proving to provide the body with the ability to heal in cases where nothing else worked. Initial findings tell us that PRP-assisted stem cells can figure out what cells they need to replicate—whether cartilage cells, bone cells, or collagen cells for ligaments and tendons—to help the body heal from within.
- Published in Blog
Supercharge your Regenerative Medicine Education


Global Stem Cells Group’s 2016 Regenerative Medicine Symposium
Global Stem Cells Group is proud to present our 2016 Edition of our Regenerative Medicine Symposium, to be held in 6 different Cities around the World. This prestigious event will have the presence of a select group of renowned international speakers who will offer a combined day of conferences of high scientific rigor aimed at Physicians.
Key Highlights of the Symposium
Global Stem Cells Group’s symposium will provide cutting-edge information on developments in all areas of stem cell research, including the biology, medicine, applications, regulations, product development, and the commercialization of stem cells.
Expert International Speakers
The symposium will feature a select group of renowned international speakers who will share their knowledge and expertise in stem cell research and applications. These experts will deliver high-level scientific presentations aimed at advancing the understanding and practice of regenerative medicine.
Comprehensive Coverage of Stem Cell Research
Attendees will gain insights into the latest advancements in stem cell biology, medical applications, regulatory landscapes, and product development. The symposium will cover a broad range of topics crucial for professionals in the field.
Business Opportunities in Stem Cell Research
Business opportunities, challenges, and potential strategies for overcoming these challenges will also be addressed. Attendees will learn about the current commercially viable categories of companies, funding strategies, and strategic relationships available within the industry.
Event Schedule and Locations
Our first event of the year will take place in Bogota, Colombia, on March 3rd. For more information and registration, click here.
- Published in Blog
Essential Steps for Regenerative Medicine Practitioners

Enhance Your Practice with Global Stem Cells Group’s Stem Cell Training Course
Global Stem Cells Group offers a stem cell training course that can help you bring some of our cutting-edge regenerative therapies to your practice or clinic. We offer an intensive, hands-on two-day training class that covers a range of essential techniques and knowledge.
Hands-On Training and Techniques
In our training course, we show you how to collect fat tissue via our precision mini lipo-aspiration technique and walk you through the process of collecting bone marrow via the iliac crest. This hands-on approach ensures you gain practical experience in performing these procedures.
Comprehensive Didactic Lectures
We provide didactic lectures on stem cell structure, function, and treatment. These lectures cover techniques to isolate and harvest stem cells from fat tissue and bone marrow, as well as platelet-rich plasma from peripheral blood. All of this is done on actual patients—three to four of them during the two-day course period—so you can gain a thorough understanding of the procedures.
Course Materials and Accreditation
GSCG will provide you with written protocols, forms, and consent documents, making it easy to implement these techniques in your practice after certification. We also offer instructional videos, quality control (QC) assays, viability and cell counts. Our courses are fully accredited, providing 16 categories for one credit, by three different universities. Additionally, we offer the opportunity to participate in Institutional Review Board (IRB) clinical studies.
FDA-Approved Bone Marrow Products
For the bone marrow product, we’ve partnered with Emcyte. All of the kits for bone marrow and platelet-rich plasma are FDA 510K approved. This approval ensures the safe collection of a small blood sample to produce concentrated growth factors and platelets. The system processes bone marrow aspirate precisely for the purest concentration of cells at the point of care.
Adipose-Derived Stem Cell Kit
Our adipose-derived stem cell kit leverages fat tissue, one of the most plentiful sources of stem cells in the body, particularly mesenchymal stem cells. Fat tissue is ideal for treating degenerative diseases or replenishing damaged tissue. This kit allows you to obtain about five hundred times more mesenchymal stem cells from fat tissue than from bone marrow.
Isolation Kits and Lab Support
Global Stem Cells Group offers a variety of isolation kits, produced according to good manufacturing processes. We have a full-scale laboratory in Santiago, Chile, where we produce all necessary reagents. Our kits include all consumables necessary to isolate regenerative stem cells. Visit Adimarket.net to view our variety of products, including centrifuges and other medical devices essential for stem cell therapies.
Equip Your Clinic with Essential Tools
During the stem cell training course, we also cover the equipment you will need to perform these techniques. Our comprehensive training ensures you are well-prepared to bring stem cell therapies to your patients, enhancing your practice with the latest advancements in regenerative medicine.
- Published in Blog
(Almost) Everything You Wanted to Know About Stem Cells But Were Afraid to Ask
Discovering the Power of Stem Cells
Stem cells have captivated the interest of biologists, healthcare professionals, and the curious public alike for years. At Global Stem Cells Group, we are pioneering stem cell treatments for a wide range of medical conditions, making them accessible in physician offices and outpatient clinics worldwide, with plans to expand availability in the U.S. soon.
Understanding Stem Cells

A stem cell is defined by its unique ability to self-renew and differentiate into specialized cells needed for various treatments. These versatile cells can transform into muscle cells, heart cells, skin cells, and more through specific chemical and genetic signals manipulated by stem cell physicians.
Sources of Stem Cells

Different types of stem cells originate from various sources. Adult adipose-derived stem cells, found abundantly in fat tissue, are particularly favored for their ease of harvest and plentiful supply. Each liter of fat yields hundreds of millions of potential stem cells, capable of becoming fat, heart, bone, or muscle tissue.
Global Stem Cells Group’s Approach
Global Stem Cells Group offers validated, compliant outpatient methods for providing adipose-derived and bone marrow-derived stem cells to physicians worldwide. We have trained over 500 physicians who now offer stem cell therapies in their practices, pioneering the integration of regenerative medicine into mainstream healthcare.
How Stem Cell Therapy Works

Stem cell therapy leverages the body’s innate healing potential to reverse the effects of aging, degenerative diseases, and injuries. By guiding stem cells to replicate youthful and vigorous cells, we rejuvenate tissues and restore function. Stem cell therapy is evolving beyond regenerative medicine, combining techniques like gene therapy and advanced biologics for more targeted treatments.
The Future of Regenerative Medicine
Regenerative medicine represents a transformative shift in healthcare, akin to historical breakthroughs like the polio vaccine and antibiotics. Combining cellular and gene therapies promises new avenues for treating previously untreatable conditions, ushering in a new era of medical innovation and patient care.
- Published in Blog
Could stem cells repair the damaged brain in Alzheimer’s?
Exploring Stem Cell Therapies for Alzheimer’s Disease
Alzheimer’s disease presents a formidable challenge, characterized by progressive cognitive decline and neurological deterioration. At Global Stem Cells Group, we delve into the potential of stem cell therapies to revolutionize Alzheimer’s treatments, offering hope where conventional methods have fallen short.
Understanding Alzheimer’s Disease
Alzheimer’s primarily affects individuals aged 70 and older, with women more susceptible. This irreversible brain disorder impacts memory, cognition, and daily functioning, posing significant challenges for patients and caregivers alike.
Current Treatment Challenges
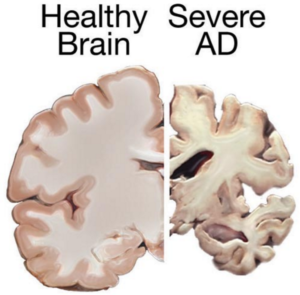
Despite extensive research, there is currently no cure for Alzheimer’s. Existing treatments focus on managing symptoms temporarily, offering limited relief from cognitive decline and behavioral changes.
The Role of Stem Cells in Alzheimer’s Treatment
Stem cell therapies offer a promising alternative by potentially replacing damaged neurons with healthy ones. Neural stem cells, for instance, could be transplanted into the brain to regenerate neurons and restore cognitive function. Challenges include ensuring cell integration and overcoming the presence of disease-causing proteins that may compromise long-term efficacy.
Potential Therapeutic Approaches
Mesenchymal Stem Cells in Alzheimer’s

Research suggests using stem cells to deliver neurotrophins, proteins crucial for neuron survival and function. Although promising results have been observed in animal studies, translating these findings to human treatments requires further exploration.
Mesenchymal stem cells show potential for their anti-inflammatory properties, which may mitigate Alzheimer’s symptoms. However, clinical studies are necessary to validate their safety and effectiveness in treating this complex neurodegenerative disorder.
Advancements in Stem Cell Research
Recent breakthroughs, such as reprogramming skin cells into induced pluripotent stem cells (iPSCs), offer new avenues for studying Alzheimer’s pathology and testing innovative treatments. These advancements provide hope for identifying novel therapeutic strategies.
Conclusion
While challenges persist in developing effective stem cell therapies for Alzheimer’s, ongoing research and clinical trials hold promise for advancing treatment options. Global Stem Cells Group remains committed to pioneering new approaches that could transform the landscape of Alzheimer’s care.
References
- http://www.eurostemcell.org/factsheet/alzheimer%E2%80%99s-disease-how-could-stem-cells-help
- http://hsci.harvard.edu/news/alzheimer%E2%80%99s-dish
- http://www.ipscell.com/2012/05/can-stem-cells-be-used-to-treat-alzheimers-disease/
- http://www.sciencedirect.com/science/article/pii/S1934590915003173
- http://www.cell.com/cell-stem-cell/abstract/S1934-5909%2815%2900305-7
- Published in Blog
Could stem cells offer the cure for muscular dystrophy?
Exploring Stem Cell Therapies for Muscular Dystrophy
Muscular dystrophy (MD) encompasses a group of genetic disorders causing progressive muscle weakness and loss of mass. At Global Stem Cells Group, we delve into the potential of stem cell treatments to revolutionize care for muscular dystrophy patients worldwide.
Understanding Muscular Dystrophy
Muscular dystrophy affects voluntary muscles, with symptoms ranging from mild to severe, impacting mobility and daily life. Duchenne muscular dystrophy (DMD), the most severe form, primarily affects young boys, leading to progressive muscle degeneration and shortened lifespan.
Challenges in Current Treatments
While treatments like physiotherapy and steroids aim to manage symptoms, there is no cure for DMD. These approaches often fall short due to side effects and limited effectiveness in halting disease progression.
The Promise of Stem Cells in Muscular Dystrophy
Stem cell therapies offer potential by addressing the underlying cause—defective dystrophin production. Research explores using stem cells from muscle tissue, bone marrow, and blood vessels to regenerate muscle fibers deficient in dystrophin, showcasing promising results in animal models.
Recent Advances and Research
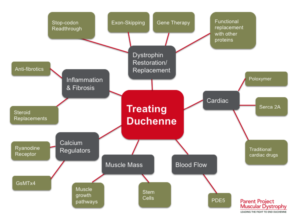
Studies have demonstrated that stem cells isolated from muscle blood vessels can restore mobility in affected animals. Researchers have also explored genetic correction combined with stem cell therapy, showing improvements in muscle regeneration and function in mice models of DMD.
Future Directions
While translating these findings to human treatments requires further research, combined therapies integrating stem cells and gene therapies show promise. These approaches aim to replace defective stem cells with healthy ones or reduce inflammation to preserve muscle function effectively.
Conclusion
While a cure for muscular dystrophy remains elusive, stem cell research holds immense promise. Global Stem Cells Group remains at the forefront, exploring innovative therapies that could transform the outlook for patients with muscular dystrophy.
References:
- http://www.mayoclinic.org/diseases-conditions/muscular-dystrophy/basics/definition/con-20021240
- http://www.eurostemcell.org/factsheet/muscular-dystrophy-how-could-stem-cells-help
- https://www.mda.org/disease/duchenne-muscular-dystrophy/research
- http://quest.mda.org/article/scientists-bullish-stem-cells-muscle-repair
- http://hsci.harvard.edu/stem-cells-used-treat-muscular-dystrophy-mice
- https://med.stanford.edu/news/all-news/2014/12/stem-cells-faulty-in-duchenne-muscular-dystrophy.html
http://www.mayoclinic.org/diseases-conditions/muscular-dystrophy/basics/definition/con-20021240
- http://www.eurostemcell.org/factsheet/muscular-dystrophy-how-could-stem-cells-help
- https://www.mda.org/disease/duchenne-muscular-dystrophy/research
- http://quest.mda.org/article/scientists-bullish-stem-cells-muscle-repair
- http://hsci.harvard.edu/stem-cells-used-treat-muscular-dystrophy-mice
- https://med.stanford.edu/news/all-news/2014/12/stem-cells-faulty-in-duchenne-muscular-dystrophy.html
- Published in Blog
Stem cells may be used for healing damaged lungs
Stem Cells: A Promising Approach for Lung Tissue Repair
Stem cell research offers new hope for treating debilitating lung conditions such as bronchitis, asthma, cystic fibrosis, and emphysema, which affect millions worldwide.
Research at the Weizmann Institute of Science
Scientists at the Weizmann Institute of Science have discovered that stem cells could potentially repair damaged lung tissues. Their study demonstrated that embryonic stem cells, similar to those found in bone marrow, can migrate to damaged lung compartments, differentiate, and regenerate lung tissue in mice models [1].
Implications of Lung-specific iPSCs
Previous studies, including research from the Boston University Medical Center, have focused on generating lung-specific induced pluripotent stem cells (iPSCs) from patients with lung diseases. These iPSCs show promise as they can be cultivated without embryos, reducing the risk of rejection in transplants. They have been found capable of differentiating into lung tissue precursor cells, offering a potential alternative to embryonic stem cells [2].
Future Directions in Stem Cell Therapy
Ongoing research aims to optimize stem cell transplantation methods for treating severe respiratory diseases. Scientists are exploring drug dosages to prevent rejection and considering the creation of stem cell banks to facilitate broader clinical applications.
Conclusion
While still in experimental stages, stem cell therapies represent a promising avenue for repairing damaged lungs and improving respiratory function. Continued research and development are crucial for translating these findings into effective treatments for patients worldwide.
References:
- Chava Rosen, Elias Shezen, Anna Aronovich, Yael Zlotnikov Klionsky et al. – Preconditioning allows engraftment of mouse and human embryonic lung cells, enabling lung repair in mice, Nature Medicine, 2015, http://www.nature.com/nm/journal/vaop/ncurrent/full/nm.3889.html
- Aba Somers, Jyh-Chang Jean, Cesar A. Sommer, Amel Omari et al. – Generation of transgene-free lung disease-specific human induced pluripotent stem cells using a single excisable lentiviral stem cell cassette, Stem Cells, 2010, 28 (10):1728, http://onlinelibrary.wiley.com/doi/10.1002/stem.495/full
- Published in Blog
Adult tissues that serve as source for stem cells
Exploring Adult Stem Cells: Sources and Potential
Stem cells derived from adult tissues are gaining attention for their regenerative potential across various medical conditions, offering hope for treating diseases like osteoarthritis, muscular dystrophy, and Alzheimer’s.
Bone Marrow: A Rich Source of Stem Cells
Bone marrow harbors hematopoietic stem cells (HSCs) capable of differentiating into various blood cell types—essential for immune function and blood clotting. Additionally, skeletal stem cells (STCs) contribute to bone and cartilage regeneration [1].
Neural Stem Cells (NSCs) in Brain Research
Neural stem cells found in the brain show promise in replacing dying neurons, highlighting potential applications in neurological disorders such as multiple sclerosis and Parkinson’s disease [2].
Intestinal Stem Cells (ISCs) and Their Role
ISCs, lining the intestines, continuously renew tissue throughout life but pose challenges in cancer contexts where they can contribute to tumor regeneration post-treatment.
Liver: A Hub of Regeneration
Hepatocytes, liver’s primary cells, exhibit stem cell-like properties, crucial for regenerating liver tissue damaged by infections or surgery. Research suggests specialized stem cells from the biliary tree could aid in diabetes treatment by generating insulin-producing islets [3].
Future Directions and Conclusion
Research into adult stem cells from diverse tissues offers insights into their therapeutic potential, bypassing ethical concerns associated with embryonic stem cells. Lower rejection risks and higher differentiation rates make them promising candidates for future regenerative medicine applications.
REFERENCES:
[1] MacKlis, Jeffrey D.; Magavi, Sanjay S.; Leavitt, Blair R. (2000). “Induction of neurogenesis in the neocortex of adult mice”. Nature 405 (6789): 951–5[2] Biliary Tree Stem Cells, Precursors to Pancreatic Committed Progenitors: Evidence for Possible Life-long Pancreatic Organogenesis – http://www.diabetesresearch.org/file/research-publications/2013-Stem-Cells_Biliary-Tree-Stem-Cells-to-Islets.pdf
- Published in Blog