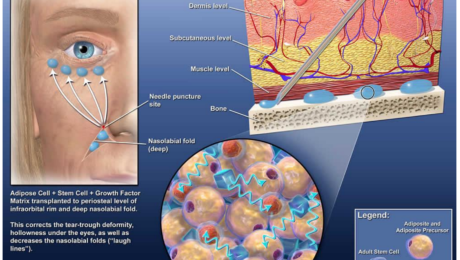
IFATS Recommendations for FDA Regulation of Human Cells, Tissues, and Cellular and Tissue-Based Products
Introduction
The International Federation of Adipose Therapeutics and Sciences (IFATS) appreciates this opportunity to submit the following comments to supplement its earlier written comments and recent testimony at the September 12-13, 2016 Public Hearing on the 2014-2015 Draft HCT/P Guidances concerning: a) Minimal Manipulation; b) Same Surgical Procedure; c) Adipose Tissue; and d) Homologous Use.
IFATS Overview
International Federation of Adipose Therapeutics and Sciences (IFATS)
45 Lyme Road – Suite 304
Hanover, NH 03755 USA
Tel: 1-603-643-2325, Fax: 1-603-643-1444
Date: September 26, 2016
Addressed to:
Division of Dockets Management (HFA–305)
Food and Drug Administration
5630 Fishers Lane, Rm. 1061
Rockville, MD 20852
Re: FDA-2014-D-1856 – Comments to 2014-2015 Draft Guidance regarding:
- Docket FDA-2014-D-1584: “Same Surgical Procedure Exception under 21 CFR 1271.15(b): Questions and Answers Regarding the Scope of the Exception; Draft Guidance for Industry”
- Docket FDA-2014-D-1696: “Minimal Manipulation of Human Cells, Tissues, and Cellular and Tissue-Based Products; Draft Guidance for Industry and Food and Drug Administration Staff”
- Docket FDA-2014-D-1856: “Human Cells, Tissues, and Cellular and Tissue-Based Products from Adipose Tissue: Regulatory Considerations; Draft Guidance for Industry”
- Docket FDA-2015-D-3581: “Homologous Use of Human Cells, Tissues, and Cellular and Tissue-Based Products; Draft Guidance for Industry and FDA Staff”
Commitment to Advancing Adipose-Based Therapies
IFATS is committed to the responsible advancement of the science and translation of new adipose therapies, ensuring patient safety. Founded in 2003 by pioneering adipose stem cell biologists and clinician–scientists, IFATS aims to advance the science of adipose tissue biology and its clinical translation to therapeutic applications.
IFATS’s Global Influence and Expertise
Membership now spans 40 countries across North America, Europe, Africa, the Middle East, Asia, Australia, and Central and South America. It includes basic scientists, translational researchers, clinicians, and regulatory and biotech representatives. IFATS is aligned with prestigious journals, Stem Cells and Stem Cells Translational Medicine, and has contributed to defining adipose-derived cells in the publication Cytotherapy.
Review of FDA Draft Guidances
Drawing on this expertise, IFATS has reviewed the 4 draft guidances with great care. It respectfully requests the FDA to reconsider and modify the 4 draft HCT/P guidances as follows:
Recommendations
Recommendation #1: Cell-Based Risks
Interpret and evaluate an HCT/P’s homologous use and minimal manipulation based on its manufacturer’s intended use in the patient.
Recommendation #2: Provider-Based Risks
Reduce provider-created risks by targeting provider behavior.
Recommendation #3: Recognize Structural and Nonstructural Functions
Recognize that adipose HCT/Ps have both structural and nonstructural functions, and regulate based on its manufacturer’s intended use in the patient.
Recommendation #4: Revise Evaluation of Minimal Manipulation and Homologous Use
Revise the evaluation of minimal manipulation and homologous use as they pertain to particular applications of adipose tissue.
Conclusion
IFATS is committed to collaborating with the FDA to meet the challenges of regulating HCT/P therapies. We respectfully request a meeting with FDA representatives to discuss these issues and others related to the advancement and regulation of adipose-based therapies.
Respectfully submitted on behalf of IFATS,
Adam J. Katz, MD, FACS
Chair, IFATS Regulatory Affairs Committee & IFATS Co-Founder
University of Florida College of Medicine
Professor
Director of Plastic Surgery Research, Laboratory of BioInnovation and Translational Therapeutics
Division of Plastic Surgery, Department of Surgery
IFATS Board of Directors
- Bruce Bunnell, PhD – Tulane University / United States
- Louis Casteilla, PhD – University of Toulouse / France
- Sydney Coleman, MD – New York & Pittsburgh Universities / United States
- Julie Fradette, PhD – Lavalle University / Canada
- William Futrell, MD – Founders’ Board, University of Pittsburgh / United States
- Marco Helder, PhD – VU University Medical Center Amsterdam / The Netherlands
- Adam J. Katz, MD, FACS – Founders’ Board, University of Florida / United States
- Ramon Llull, MD, PhD – Founders’ Board, University of Barcelona / Spain
- Kacey Marra, PhD – University of Pittsburgh / United States
- Ricardo Rodriguez, MD – President (2016), Private Practice / Johns Hopkins / United States
- Peter Rubin, MD, FACS – Chair, Founders’ Board, Chairman of the Board, University of Pittsburgh / United States
- Stuart K. Williams, PhD – University of Louisville / United States
Members-at-Large
- Jeff Gimble, MD, PhD – Pennington Biomedical / United States
- Keith March, MD, PhD – Indiana University / United States
IFATS Recommendations for FDA Regulation of Human Cells, Tissues, and Cellular and Tissue-Based Products
Introduction
IFATS recognizes the FDA’s challenge in developing regulations that fulfill the agency’s dual and interrelated responsibilities of protecting patients while promoting innovation. Although these are complementary rather than competing objectives, they are often difficult to pursue simultaneously. The FDA’s 3-tiered, risk-based §§ 361 – 351 framework balances these concerns by making the degree of regulatory oversight proportionate to the degree of an HCT/P therapy’s risk.
Key Regulatory Concepts
The concepts of homologous use and minimal manipulation are key determinants of whether an HCT/P will be classified as a § 361 product (which does not need premarket approval) or a § 351 drug, device, and/or biological product (which requires formal premarket approval). The applicability of § 351’s “same surgical procedure” exception also turns on homologous use and minimal manipulation.
Challenges for Manufacturer-Clinicians
For most manufacturer-clinicians, § 351 categorization raises insurmountable obstacles due to the time and expense of obtaining premarket approval. In such cases, § 351 classification effectively prohibits access to safe and effective HCT/P therapies, even when those therapies involve a patient’s own cells and/or can deliver superior results with reduced risks. At the same time, § 351 oversight is essential for therapies that pose greater risks due to the HCT/P’s characteristics, mechanism(s) of action, and circumstances of use.
Addressing Provider Misconduct
A second type of risk involves rogue clinicians offering false promises in the form of unproven therapies performed with few safeguards and less training. Provider misconduct is not unique to HCT/P therapies; it pervades all areas of medical practice. Nevertheless, IFATS shares the FDA’s alarm over such practices in the context of HCT/Ps and is equally determined to curtail them. Effective regulation of HCT/P-related risks must recognize and respond to their multivariate causes. Put simply:
- Sections 351 and 361 appropriately attempt to regulate HCT/P therapies proportionate to the risks of unpredictable and/or unsafe cell behavior.
- However, the risks of untrained providers misusing HCT/P therapies are caused by providers misbehaving, not cells misbehaving.
Comprehensive Risk Management Strategy
Interpretive guidance that restricts the definition and application of HCT/P terminology can only go so far in restricting provider-based risks. Additionally, restrictive, inaccurate, or imprecise definitions and interpretations carry their own risks of restricting access to therapies and a patient’s right to evaluate risk through the process of informed consent. Therefore, IFATS recommends that the FDA adopt an overall two-part strategy that focuses on both categories of HCT/P risks: those relating to cell behavior and those that pertain to provider behavior.
Recommendations
Recommendation #1 – Cell-Based Risks
Interpret and evaluate an HCT/P’s homologous use and minimal manipulation based on its manufacturer’s intended use in the patient. Interpretive guidance should predicate each definition on the functions and/or characteristics of the specific composition (i.e., cell type(s) and/or matrix or other component(s)) that are involved in, and/or relevant to, the manufacturer-clinician’s intended use in the patient.
Recommendation #2 – Provider-Based Risks
To reduce provider-created risks, the FDA should target provider behavior by collaborating with IFATS and comparable organizations to draw on and supplement existing federal and state methods of certification, registration, and similar measures.
Detailed Explanation of Recommendations
Recommendation #1 – Cell-Based Risks
The four draft guidances on homologous use, minimal manipulation, same surgical procedure, and adipose tissue individually and collectively intend to “improve stakeholders’ understanding” of 21 CFR 1271 by clarifying the FDA’s interpretation of homologous use and minimal manipulation. As demonstrated by the initial round of public comments and the ensuing public hearing on September 12 and 13, 2016, the draft guidance documents have not clarified applicable regulations. They have instead compounded the difficulty of understanding and complying with them. The drafts’ introduction of new definitional inaccuracies has also amplified rather than reduced patient risk.
IFATS respectfully requests the agency to clarify the definitions and application of homologous use and minimal manipulation by interpreting each as referring to the characteristics of the specific cell type(s) and/or the matrix or other component(s) that are involved in, and/or relevant to, the manufacturer’s intended use in the patient.
Homologous Use Definition:
21 CFR 1271.3(c): Homologous use means the repair, reconstruction, replacement, or supplementation of a recipient’s cells or tissues with an HCT/P that performs the same basic function or functions in the recipient as in the donor.
Recommended Guidance:
As used in this section, “performs the same basic function or functions in the recipient as in the donor” shall be interpreted as referring to one or more of the functions of the specific composition of the therapeutic/product, reflecting the specific cell type(s) and/or the specific matrix or other component(s) in the donor tissue that are involved in, and/or relevant to, the manufacturer’s intended use in the patient.
Minimal Manipulation Definition:
21 CFR 1271.3(f) Minimal manipulation means:
- For structural tissue, processing that does not alter the original relevant characteristics of the tissue relating to the tissue’s utility for reconstruction, repair, or replacement;
- For cells or nonstructural tissues, processing that does not alter the relevant biological characteristics of cells.
Recommended Guidance:
As used in this section, “relevant” characteristics shall be interpreted to mean the characteristics of the specific cell type(s) and/or the specific matrix or other component(s) in the donor tissue that are involved in, and/or relevant to, the manufacturer’s intended use in the patient.
Rationale:
Incorporating and relying on the manufacturer’s intended use harmonizes the interpretation and definition of homologous use and minimal manipulation with statutory directives to predicate the regulation of drugs, devices, and biologics on the manufacturer’s intended use. Defining relevant characteristics in terms of “the characteristics of specific cell type(s) and/or the matrix or other component(s) in the donor tissue that are involved in, and/or relevant to the manufacturer’s intended use in the patient” promotes patient safety by insisting on a reasonable and scientifically supportable rationale for using an HCT/P for a particular mechanism of action. This clarification balances the FDA’s dual responsibilities of protecting patients from undue safety risks while promoting the ongoing availability and continued development of HCT/P therapies.
Example of Non-Homologous Use:
Decellularized adipose matrix used to accomplish the manufacturer’s intended use of a particular metabolic or systemic effect in the patient (e.g., reducing insulin levels in a diabetic patient) is non-homologous because decellularized matrix is not relevant to metabolic or systemic activity.
Conclusion
Adopting this two-part strategy can control risk more comprehensively—and therefore more effectively—in furtherance of the FDA’s dual and interrelated obligations of protecting patients and promoting the availability of HCT/P therapies.
Introduction to FDA Regulations and IFATS Recognition
IFATS acknowledges the FDA’s dual role in patient protection and innovation promotion within the HCT/P sector. Balancing these objectives is crucial yet challenging.
Understanding the FDA’s Risk-Based Framework
The FDA’s § 361 – § 351 framework categorizes HCT/P therapies based on risk levels, influenced by concepts like homologous use and minimal manipulation.
Impact of Regulatory Classification on Access to HCT/P Therapies
Homologous use and minimal manipulation determine whether an HCT/P falls under § 361 (no premarket approval needed) or § 351 (requires premarket approval), affecting accessibility and innovation.
Provider Misconduct Risks in HCT/P Therapies
Rogue clinicians offering unproven therapies pose significant risks. Addressing provider behavior is essential for patient safety and regulatory efficacy.
IFATS Recommendations for Risk Mitigation
Recommendation #1 – Cell-Based Risks: Interpreting Homologous Use and Minimal Manipulation
IFATS proposes clarifying homologous use and minimal manipulation definitions based on manufacturer-intended use, enhancing regulatory clarity and patient safety.
Recommendation #2 – Provider-Based Risks: Targeting Provider Behavior
Collaboration with IFATS and other bodies to enhance certification and monitoring mechanisms can mitigate risks associated with provider misconduct effectively.
Recommendation #3 – Regulatory Scope for Adipose HCT/Ps
Expanding the definition of adipose tissue to include both structural and nonstructural functions aligns with biological accuracy and regulatory intent.
Conclusion: Enhancing Patient Safety and Access to HCT/P Therapies
IFATS urges the FDA to adopt a comprehensive strategy that addresses both cell-based and provider-based risks to uphold patient safety and foster innovation in HCT/P therapies.
Regulating an HCT/P’s Risks Based on Manufacturer’s Intended Use
Regulating the risks of Human Cells, Tissues, and Cellular and Tissue-Based Products (HCT/Ps) is crucially tied to their intended use and mechanisms of action in patients. This ensures effective regulatory oversight and evaluation.
Regulatory Framework: §§ 351-361
The regulatory oversight of HCT/Ps under §§ 351-361 hinges on assessing the product’s risk level. Central to this determination are the criteria of homologous use and minimal manipulation.
Homologous Use Defined (21 CFR 1271.3(c))
Homologous use is defined as the repair, reconstruction, replacement, or supplementation of a recipient’s cells or tissues with an HCT/P that performs the same basic function as in the donor.
Minimal Manipulation Criteria (21 CFR 1271.3(f))
Minimal manipulation of structural tissue involves processing that preserves the tissue’s original characteristics essential for its utility in repair, reconstruction, or replacement. For nonstructural tissues, it preserves relevant biological characteristics.
Impact on Adipose Tissue
The classification of adipose tissue as exclusively structural neglects its nonstructural functions, limiting evaluation under § 361 criteria and obstructing risk assessment.
Same Surgical Procedure Exception
The § 351 “same surgical procedure” exception applies only to HCT/Ps meeting homologous use and minimal manipulation criteria, impacting nonstructural adipose applications.
Recommendation #4: Revising Evaluation Criteria
IFATS urges the FDA to reconsider specific adipose tissue applications concerning homologous use and minimal manipulation criteria.
Example A: Decellularizing Adipose Tissue
Decellularization of adipose tissue for structural use should be recognized as minimal manipulation under §§ 351 and 361 guidelines.
Example B: Structural Use of Fat in Breast Surgery
Applying adipose tissue for breast augmentation should be considered homologous use due to its structural function in restoring form and shape.
Example C: Stromal Vascular Fraction (SVF) for Nonstructural Use
SVF extraction from adipose tissue retains nonstructural components crucial for nonstructural applications, meeting minimal manipulation and homologous use criteria.
Conclusion and Call to Action
IFATS requests the FDA to amend draft guidance on HCT/Ps to align with scientific understanding and clinical practices of adipose tissue applications.
References
- Bourin P, Bunnell BA, Casteilla L, Dominici M, Katz AJ, March KL, Redl H, Rubin JP, Yoshimura K, Gimble Stromal cells from the adipose tissue-derived stromal vascular fraction and culture expanded adipose tissue-derived stromal/stem cells: A joint statement of the International Federation for Adipose Therapeutics and Science (IFATS) and the International Society for Cellular Therapy (ISCT). Cytotherapy. 2013;15:641-648
- Diaz-Flores L, Gutierrez R, Madrid JF, Varela H, Valladares F, Acosta E, Martin-Vasallo P, Diaz-Flores L, Pericytes. Morphofunction, interactions and pathology in a quiescent and activated mesenchymal cell niche. Histol Histopathol. 2009;24:909-969
- Gimble The function of adipocytes in the bone marrow stroma. The New Biologist. 1990;2:304-312
- Cawthorn WP, Scheller EL, Learman BS, Parlee SD, Simon BR, Mori H, Ning X, Bree AJ, Schell B, Broome Bone marrow adipose tissue is an endocrine organ that contributes to increased circulating adiponectin during caloric restriction. Cell metabolism. 2014;20:368-375
- Meunier P, Aaron J, Edouard C, VlGNON Osteoporosis and the replacement of cell populations of the marrow by adipose tissue: A quantitative study of 84 iliac bone biopsies. Clinical orthopaedics and related research. 1971;80:147-154
- N. Über die wiederanheilung vollstädig vom körper getrennter, die ganze fettschicht en- thaltender hautstucke. Zbl f Chir 1893;30:16-17
- Hollander E, Joseph Cosmetic surgery. Handbuch der Kosmetik. Leipzig, Germany: Veriag von Velt. 1912;688
- Miller Cannula implants and review of implantation technics in esthetic surgery: In two parts. Oak Press; 1926.
- Gimble JM Fat circadian biology. Journal of applied physiology. 2009;107:1629-1637
- Tartaglia LA, Dembski M, Weng X, Deng N, Culpepper J, Devos R, Richards GJ, Campfield LA, Clark FT, Deeds Identification and expression cloning of a leptin receptor, ob-r. Cell. 1995;83:1263-1271
- Salgado AJ, Gimble Secretome of mesenchymal stem/stromal cells in regenerative medicine.
Biochimie. 2013;95:2195
- Salgado AJ, Reis RL, Sousa N, Gimble Adipose tissue derived stem cells secretome: Soluble factors and their roles in regenerative medicine. Curr Stem Cell Res Ther. 2009
- Khan M, Joseph Adipose tissue and adipokines: The association with and application of adipokines in obesity. Scientifica. 2014;2014
- Vicennati V, Garelli S, Rinaldi E, Di Dalmazi G, Pagotto U, Pasquali Cross-talk between
adipose tissue and the hpa axis in obesity and overt hypercortisolemic states. Hormone molecular biology and clinical investigation. 2014;17:63-77
- Kargi AY, Iacobellis Adipose tissue and adrenal glands: Novel pathophysiological mechanisms and clinical applications. International journal of endocrinology. 2014;2014
- Maïmoun L, Georgopoulos NA, Sultan Endocrine disorders in adolescent and young female athletes: Impact on growth, menstrual cycles, and bone mass acquisition. The Journal of Clinical Endocrinology
& Metabolism. 2014;99:4037-4050
- McIntosh K, Zvonic S, Garrett S, Mitchell JB, Floyd ZE, Hammill L, Kloster A, Di Halvorsen Y, Ting JP,
Storms RW. The immunogenicity of human adipose‐derived cells: Temporal changes in vitro. Stem cells. 2006;24:1246-1253
- McIntosh KR, Frazier T, Rowan BG, Gimble Evolution and future prospects of adipose- derived immunomodulatory cell therapeutics. Expert review of clinical immunology. 2013;9:175-184
- McIntosh KR, Lopez MJ, Borneman JN, Spencer ND, Anderson PA, Gimble Immunogenicity of allogeneic adipose-derived stem cells in a rat spinal fusion model. Tissue Engineering Part A. 2009;15:2677-2686
- Mitchell JB, McIntosh K, Zvonic S, Garrett S, Floyd ZE, Kloster A, Di Halvorsen Y, Storms RW, Goh B, Kilroy G. Immunophenotype of human adipose‐derived cells: Temporal changes in stromal‐associated and stem cell–associated markers. Stem cells. 2006;24:376-385
- Gimble JM, Dorheim MA, Cheng Q, Medina K, Wang CS, Jones R, Koren E, Pietrangeli C, Kincade Adipogenesis in a murine bone marrow stromal cell line capable of supporting b lineage lymphocyte growth and proliferation: Biochemical and molecular characterization. European journal of immunology. 1990;20:379-387
- Frazier TP, McLachlan JB, Gimble JM, Tucker HA, Rowan Human adipose-derived stromal/stem cells induce functional cd4+ cd25+ foxp3+ cd127− regulatory t cells under low oxygen culture conditions. Stem cells and development. 2014;23:968-977
- Frazier TP, Gimble JM, Kheterpal I, Rowan Impact of low oxygen on the secretome of human adipose- derived stromal/stem cell primary cultures. Biochimie. 2013;95:2286-2296
- Miranville A, Heeschen C, Sengenes C, Curat C, Busse R, Bouloumie Improvement of postnatal neovascularization by human adipose tissue-derived stem cells. Circulation. 2004;110:349-355
- Rehman J, Traktuev D, Li J, Merfeld-Clauss S, Temm-Grove CJ, Bovenkerk JE, Pell CL, Johnstone BH, Considine RV, March Secretion of angiogenic and antiapoptotic factors by human adipose stromal cells. Circulation. 2004;109:1292-1298
- Planat-Benard V, Silvestre J-S, Cousin B, André M, Nibbelink M, Tamarat R, Clergue M, Manneville C, Saillan-Barreau C, Duriez Plasticity of human adipose lineage cells toward endothelial cells physiological and therapeutic perspectives. Circulation. 2004;109:656-663
- Kilroy GE, Foster SJ, Wu X, Ruiz J, Sherwood S, Heifetz A, Ludlow JW, Stricker DM, Potiny S, Green P, Halvorsen YD, Cheatham B, Storms RW, Gimble Cytokine profile of human adipose-derived stem cells: Expression of angiogenic, hematopoietic, and pro- inflammatory factors. J Cell Physiol. 2007;212:702-709
- Ribeiro CA, Fraga JS, Grãos M, Neves NM, Reis RL, Gimble JM, Sousa N, Salgado The secretome of stem cells isolated from the adipose tissue and wharton jelly acts differently on central nervous system derived cell populations. Stem Cell Res Ther. 2012;3:18
- Silva NA, Gimble JM, Sousa N, Reis RL, Salgado Combining adult stem cells and olfactory ensheathing cells: The secretome effect. Stem cells and development. 2013;22:1232-1240
- Cho YJ, Song HS, Bhang S, Lee S, Kang BG, Lee JC, An J, Cha CI, Nam DH, Kim. Therapeutic effects of human adipose stem cell‐conditioned medium on stroke. Journal of neuroscience research. 2012;90:1794-1802
- Egashira Y, Sugitani S, Suzuki Y, Mishiro K, Tsuruma K, Shimazawa M, Yoshimura S, Iwama T, Hara The conditioned medium of murine and human adipose-derived stem cells exerts neuroprotective effects against experimental stroke model. Brain research. 2012;1461:87-95
- Wei X, Du Z, Zhao L, Feng D, Wei G, He Y, Tan J, Lee WH, Hampel H, Dodel Ifats collection:The conditioned media of adipose stromal cells protect against hypoxia‐ischemia‐induced brain damage in neonatal rats. Stem Cells. 2009;27:478-488
- Wei X, Zhao L, Zhong J, Gu H, Feng D, Johnstone B, March K, Farlow M, Du Adipose stromal cells-secreted neuroprotective media against neuronal apoptosis. Neuroscience letters. 2009;462:76-79
- Zhao L, Wei X, Ma Z, Feng D, Tu P, Johnstone B, March K, Du Adipose stromal cells- conditional medium protected glutamate-induced cgns neuronal death by bdnf. Neuroscience letters. 2009;452:238-240
- Cousin B, André M, Arnaud E, Pénicaud L, Casteilla Reconstitution of lethally irradiated mice by cells isolated from adipose tissue. Biochemical and biophysical research communications. 2003;301:1016-1022
- Han J, Koh YJ, Moon HR, Ryoo HG, Cho CH, Kim I, Koh Adipose tissue is an extramedullary reservoir for functional hematopoietic stem and progenitor cells. Blood.2009
- Harms M, Seale Brown and beige fat: Development, function and therapeutic potential. Nature medicine. 2013;19:1252-1263
- Rahman S, Lu Y, Czernik PJ, Rosen CJ, Enerback S, Lecka-Czernik Inducible brown adipose tissue, or beige fat, is anabolic for the skeleton. Endocrinology. 2013;154:2687-2701
- Wu J, Cohen P, Spiegelman Adaptive thermogenesis in adipocytes: Is beige the new brown? Genes & development. 2013;27:234-250
- Krings A, Rahman S, Huang S, Lu Y, Czernik P, Lecka-Czernik Bone marrow fat has brown adipose tissue characteristics, which are attenuated with aging and diabetes. Bone. 2012;50:546-552
- van Marken Lichtenbelt WD, Vanhommerig JW, Smulders NM, Drossaerts JM, Kemerink GJ, Bouvy ND, Schrauwen P, Teule Cold-activated brown adipose tissue in healthy men. N Engl J Med. 2009;360:1500-1508
- Peirce V, Carobbio S, Vidal-Puig The different shades of fat. Nature. 2014;510:76-83
- Enerbäck S, Gimble Lipoprotein lipase gene expression: Physiological regulators at the transcriptional and post-transcriptional level. Biochimica et Biophysica Acta (BBA)- Lipids and Lipid Metabolism. 1993;1169:107-125
- Rudolph MC, Neville MC, Anderson Lipid synthesis in lactation: Diet and the fatty acid switch. Journal of mammary gland biology and neoplasia. 2007;12:269-281
- Gimble JM, Katz AJ, Bunnell Adipose-derived stem cells for regenerative medicine. Circ Res. 2007;100:1249-1260
- Bellows CF, Zhang Y, Chen J, Frazier ML, Kolonin Circulation of progenitor cells in obese and lean colorectal cancer patients. Cancer Epidemiology Biomarkers & Prevention. 2011;20:2461- 2468
- Bellows CF, Zhang Y, Simmons PJ, Khalsa AS, Kolonin Influence of bmi on level of circulating progenitor cells. Obesity. 2011;19:1722-1726
- Krijnen PA NB, Meinster E, Vo K, Musters RJ, Kamp O, Niessen HW,, Juffermans LJ. Acute myocardial infarction does not affect functional characteristics of adipose derived stem cells in rats, but reduces the number of stem cells in adipose tissue. IFATS Annual Meeting. 2014:100
- Traktuev DO, Merfeld-Clauss S, Li J, Kolonin M, Arap W, Pasqualini R, Johnstone BH,March KL. A population of multipotent cd34-positive adipose stromal cells share pericyte and mesenchymal surface markers, reside in a periendothelial location, and stabilize endothelial networks. Circulation research. 2008;102:77-85
- Traktuev DO, Prater DN, Merfeld-Clauss S, Sanjeevaiah AR, Saadatzadeh MR, Murphy M, Johnstone BH, Ingram DA, March Robust functional vascular network formation in vivo by cooperation of adipose progenitor and endothelial cells. Circulation research. 2009;104:1410-1420
- Merfeld-Clauss S, Gollahalli N, March KL, Traktuev Adipose tissue progenitor cells directly interact with endothelial cells to induce vascular network formation. Tissue Engineering Part A. 2010;16:2953-2966
- Merfeld-Clauss S, Lupov IP, Lu H, Feng D, Compton-Craig P, March KL, Traktuev. Adipose stromal cells differentiate along a smooth muscle lineage pathway upon endothelial cell contact via induction of activin a. Circulation research. 2014;115:800-809
- Crisan M, Yap S, Casteilla L, Chen C-W, Corselli M, Park TS, Andriolo G, Sun B, Zheng B, Zhang A perivascular origin for mesenchymal stem cells in multiple human organs. Cell stem cell. 2008;3:301-313
- Ter Horst E, Naaijkens B, Krijnen P, Van Der Laan A, Piek J, Niessen Induction of a monocyte/macrophage phenotype switch by mesenchymal stem cells might contribute to improved infarct healing postacute myocardial infarction. Minerva cardioangiologica. 2013;61:617-625
- Guisantes E, Fontdevila J, Rodríguez Autologous fat grafting for correction of unaesthetic scars. Annals of plastic surgery. 2012;69:550-554
- Klinger M, Caviggioli F, Klinger FM, Giannasi S, Bandi V, Banzatti B, Forcellini D, Maione L, Catania B, Vinci Autologous fat graft in scar treatment. Journal of Craniofacial Surgery. 2013;24:1610-1615
- Klinger M, Marazzi M, Vigo D, Torre Fat injection for cases of severe burn outcomes: A new perspective of scar remodeling and reduction. Aesthetic plastic surgery. 2008;32:465-469
- Khouri RK, Smit JM, Cardoso E, Pallua N, Lantieri L, Mathijssen IM, Khouri Jr RK, Rigotti Percutaneous aponeurotomy and lipofilling: A regenerative alternative to flap reconstruction? Plastic and reconstructive surgery. 2013;132:1280-1290
- Balkin DM, Samra S, Steinbacher Immediate fat grafting in primary cleft lip repair. Journal of Plastic, Reconstructive & Aesthetic Surgery. 2014;67:1644-1650
- Rigotti G, Marchi A, Galie M, Baroni G, Benati D, Krampera M, Pasini A, Sbarbati. Clinical treatment of radiotherapy tissue damage by lipoaspirate transplant: A healing process mediated by adipose-derived adult stem cells. Plastic and reconstructive surgery. 2007;119:1409-1422
- Villani F, Caviggioli F, Klinger F, Klinger Rehabilitation of irradiated head and neck tissues by autologous fat transplantation. Plastic and reconstructive surgery. 2009;124:2190-2191
- Chang CC, Thanik VD, Lerman OZ, Saadeh PB, Warren SM, Coleman SR, Hazen Treatment of radiation skin damage with coleman fat grafting. STEM CELLS. 2007;25:3280-3281
- Sultan SM, Stern CS, Allen Jr RJ, Thanik VD, Chang CC, Nguyen PD, Canizares O, Szpalski C, Saadeh PB, Warren Human fat grafting alleviates radiation skin damage in a murine model. Plastic and reconstructive surgery. 2011;128:363-372
- Loder S, Peterson JR, Agarwal S, Eboda O, Brownley C, DeLaRosa S, Ranganathan K, Cederna P, Wang SC, Levi Wound healing after thermal injury is improved by fat and adipose-derived stem cell isografts. Journal of Burn Care & Research. 2015;36:70-76
- Sultan SM, Barr JS, Butala P, Davidson EH, Weinstein AL, Knobel D, Saadeh PB, Warren SM, Coleman SR, Hazen Fat grafting accelerates revascularisation and decreases fibrosis following thermal injury. Journal of Plastic, Reconstructive & Aesthetic Surgery. 2012;65:219-227
- Cuomo R, Zerini I, Botteri G, Barberi L, Nisi G, D’ANIELLO Postsurgical pain related to breast implant: Reduction with lipofilling procedure. In Vivo. 2014;28:993-996
- Maione L, Vinci V, Caviggioli F, Klinger F, Banzatti B, Catania B, Lisa A, Klinger Autologous fat graft in postmastectomy pain syndrome following breast conservative surgery and radiotherapy. Aesthetic plastic surgery. 2014;38:528-532
- Caviggioli F, Maione L, Forcellini D, Klinger F, Klinger Autologous fat graft in postmastectomy pain syndrome. Plastic and reconstructive surgery. 2011;128:349-352
- Caviggioli F, Vinci V, Codolini Autologous fat grafting: An innovative solution for the treatment of post-mastectomy pain syndrome. Breast Cancer. 2013;20:281-282
- Salgarello M, Visconti The role of sacrolumbar fat grafting in the treatment of spinal fusion instrumentation-related chronic low back pain: A preliminary report. Spine. 2014;39:E360-E362
- Faroni A, Terenghi G, Reid Adipose-derived stem cells and nerve regeneration: Promises and pitfalls. Int Rev Neurobiol. 2013;108:121-136
- Vaienti L, Gazzola R, Villani F, Parodi Perineural fat grafting in the treatment of painful neuromas. Techniques in hand & upper extremity surgery. 2012;16:52-55
- Marangi GF, Pallara T, Cagli B, Schena E, Giurazza F, Faiella E, Zobel BB, Persichetti Treatment of early-stage pressure ulcers by using autologous adipose tissue grafts. Plastic Surgery International. 2014;2014
- Lolli P, Malleo G, Rigotti Treatment of chronic anal fissures and associated stenosis by autologous adipose tissue transplant: A pilot study. Diseases of the Colon & Rectum. 2010;53:460-466
- Cantarella G, Baracca G, Forti S, Gaffuri M, Mazzola Outcomes of structural fat grafting for paralytic and non-paralytic dysphonia. Acta Otorhinolaryngologica Italica. 2011;31:154
- DeFatta RA, DeFatta RJ, Sataloff Laryngeal lipotransfer: Review of a 14-year experience. Journal of Voice. 2013;27:512-515
- Sataloff Autologous fat implantation for vocal fold scar. Current opinion in otolaryngology & head and neck surgery. 2010;18:503-506
- Cantarella G, Mazzola RF, Mantovani M, Baracca G, Pignataro Treatment of velopharyngeal insufficiency by pharyngeal and velar fat injections. Otolaryngology– Head and Neck Surgery. 2011;145:401-403
- Papa N, Luca G, Sambataro D, Zaccara E, Maglione W, Gabrielli A, Fraticelli P, Moroncini G, Beretta L, Santaniello A. Regional implantation of autologous adipose tissue-derived cells induces a prompt healing of long-lasting indolent digital ulcers in patients with systemic sclerosis. Cell transplantation. 2014
- Hovius SE, Kan HJ, Smit X, Selles RW, Cardoso E, Khouri Extensive percutaneous aponeurotomy and lipografting: A new treatment for dupuytren disease. Plastic and reconstructive surgery. 2011;128:221-228
- Verhoekx JS, Mudera V, Walbeehm ET, Hovius Adipose-derived stem cells inhibit the contractile myofibroblast in dupuytren’s disease. Plastic and reconstructive surgery. 2013;132:1139-1148
- Bank J, Fuller SM, Henry GI, Zachary Fat grafting to the hand in patients with raynaud phenomenon: A novel therapeutic modality. Plastic and reconstructive surgery. 2014;133:1109-1118
- Damgaard OE, Siemssen Lipografted tenolysis. Journal of Plastic, Reconstructive & Aesthetic Surgery. 2010;63:e637-e638
- Colonna M, Scarcella M, d’Alcontres F, Delia G, Lupo Should fat graft be recommended in tendon scar treatment? Considerations on three cases (two feet and a severe burned hand). European review for medical and pharmacological sciences. 2014;18:753-759
- Merikanto JE, Alhopuro S, Ritsilä Free fat transplant prevents osseous reunion of skull defects: A new approach in the treatment of craniosynostosis. Scandinavian Journal of Plastic and Reconstructive Surgery and Hand Surgery. 1987;21:183-188
- Mojallal A, Lequeux C, Shipkov C, Breton P, Foyatier J-L, Braye F, Damour Improvement of skin quality after fat grafting: Clinical observation and an animal study. Plastic and reconstructive surgery. 2009;124:765-774
- Lockwood Superficial fascial system (sfs) of the trunk and extremities: A new concept. Plastic and reconstructive surgery. 1991;87:1009-1018
- Song AY, Askari M, Azemi E, Alber S, Hurwitz DJ, Marra KG, Shestak KC, Debski R, Rubin Biomechanical properties of the superficial fascial system. Aesthetic Surgery Journal. 2006;26:395-403
- Flynn The use of decellularized adipose tissue to provide an inductive microenvironment for the adipogenic differentiation of human adipose-derived stem cells. Biomaterials. 2010;31:4715-4724
- Brown BN, Freund JM, Han L, Rubin JP, Reing JE, Jeffries EM, Wolf MT, Tottey S, Barnes CA, Ratner Comparison of three methods for the derivation of a biologic scaffold composed of adipose tissue extracellular matrix. Tissue Engineering Part C: Methods. 2011;17:411-421
- Wu I, Nahas Z, Kimmerling KA, Rosson GD, Elisseeff An injectable adipose matrix for soft tissue reconstruction. Plastic and reconstructive surgery. 2012;129:1247
- Omidi E, Fuetterer L, Mousavi SR, Armstrong RC, Flynn LE, Samani Characterization and assessment of hyperelastic and elastic properties of decellularized human adipose tissues. Journal of biomechanics. 2014;47:3657-3663
- Wang L, Johnson JA, Zhang Q, Beahm Combining decellularized human adipose tissue extracellular matrix and adipose-derived stem cells for adipose tissue engineering. Acta biomaterialia. 2013;9:8921-8931
- Healy C, Allen Sr The evolution of perforator flap breast reconstruction: Twenty years after the first diep flap. Journal of reconstructive microsurgery. 2014;30:121-125
- LoTempio MM, Allen Breast reconstruction with sgap and igap flaps. Plastic and reconstructive surgery. 2010;126:393-401
- Erić M, Mihić N, Krivokuća Breast reconstruction following mastectomy; patient’s satisfaction. Acta Chir Belg. 2009;109:159-166
- Diaz-Flores L, Gutierrez R, Lizartza K, et Behavior of In Situ Human Native Adipose Tissue CD34+ Stromal/Progenitor Cells During Different Stages of Repair. Tissue-Resident CD34+ Stromal Cells as a Source of Myofibroblasts. Anatomical record. 2014.
- Gil-Ortega M, Garidou L, Barreau C, et Native adipose stromal cells egress from adipose tissue in vivo: evidence during lymph node activation. Stem cells. 2013;31(7):1309-20.

- Published in Blog
Purest Liver-like Cells to Date Generated from Induced Pluripotent Stem Cells (iPSCs)
SATURDAY, 01 OCTOBER 2016 / PUBLISHED IN BLOG
Researchers from the Medical University of South Carolina (MUSC) and the University of Pennsylvania have discovered a new methodology for purifying liver cells generated from induced pluripotent stem cells (iPSCs) that could facilitate progress toward an important clinical goal: treating patients with disease-causing liver mutations by transplanting unmutated liver cells derived from their own stem cells.
Background on Liver Cell Generation from iPSCs
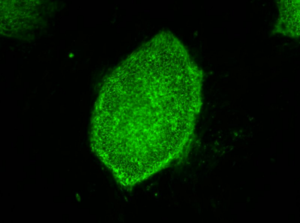
Credit: Image courtesy of Stephen A. Duncan, Ph.D., at the Medical University of South Carolina
Previous attempts to generate liver-like cells from stem cells have yielded heterogeneous cell populations with little similarity to diseased livers in patients.
The Role of the Next Generation Genetic Association Studies Program
The National Heart, Lung, and Blood Institute (NHLBI)’s Next Generation Genetic Association Studies Program (Next Gen) was created to bank stem cell lines sourced from patients in genome-wide association studies (GWAS). The goal of the Next Gen Lipid Conditions sub-section, a collaborative effort between Stephen A. Duncan, Ph.D., chair of regenerative medicine at MUSC, and Daniel J. Rader, M.D., and Edward E. Morrisey, Ph.D., both at the University of Pennsylvania, is to help determine the genetic sources of heart, lung, or blood conditions that also include the liver.
Genome-Wide Association Studies (GWAS)
GWAS studies map the genomes in hundreds of people to look for genetic mutation patterns that differ from the genomes of healthy individuals. As GWAS studies map more genomes, they become more likely to find the correct genetic mutations that cause a disease. Once a panel of suspected mutations is built, stem cells from these individuals can be manipulated in culture dishes to differentiate into any of the body’s cells. The cells can be screened to learn more about the mutations and to test panels of drugs that might ultimately help treat patients harboring a disease.
Challenges in Cell Manipulation Process
Problems arise during the cell manipulation process. For example, iPSCs persistently refuse to mature uniformly into liver-like cells when fed growth factors. Traditionally, antibodies have been used to recognize features of maturity on the surfaces of cells and purify cells that are similar, an approach that has been crucial to stem cell research. But available antibodies that recognize mature liver cells are scanty and tend to recognize many different kinds of cells. The many types of cells in mixed populations have diverse characteristics that can obscure underlying disease-causing genetic variations, which tend to be subtle.
Introduction of Chemo Proteomic Cell Surface Capture (CSC) Technology
Instead of relying on antibodies, Duncan and his team embraced a new technology called chemo proteomic cell surface capture (CSC) technology. CSC technology allowed the researchers to map the most highly produced proteins on the surface of liver cells during the final stages of differentiation of stem cells into liver cells. The most abundant protein was targeted with an antibody labeled with a fluorescent marker and used to sort the mature liver cells from the rest.
Successful Generation of Pure Liver-like Cells
The procedure was highly successful: The team had a population of highly pure, homogeneous, and mature liver-like cells. Labeled cells had far more similar traits of mature hepatocytes than unlabeled cells. Pluripotent stem cells that had not differentiated were excluded from the group of labeled cells.
“That’s important,” says Duncan. “If you’re wanting to transplant cells into somebody that has liver disease, you really don’t want to be transplanting pluripotent cells because pluripotent cells form tumors called teratocarcinomas.”
Future Implications
Duncan cautioned that transplantation of iPSC-derived liver cells is not yet ready for translation to the clinic, but the technology for sorting homogeneous liver cells can be used now to successfully and accurately model and study disease in the cell culture dish.
“We think that the ability to generate pure populations will get rid of the variability, and therefore really help us combine with GWAS studies to identify allelic variations that are causative of a disease, at least in the liver,” he says.
Contributions to the Study
Researchers at the University of Minnesota (Minneapolis) and the Medical College of Wisconsin (Milwaukee) contributed to the study, published August 25, in Stem Cell Reports.
- Published in Blog
Breakthrough in scaling up life-changing stem cell production
SATURDAY, 01 OCTOBER 2016 / PUBLISHED IN BLOG
Scientists from the U.K. and Sweden have achieved a significant breakthrough in creating human stem cells that addresses the challenge of large-scale production, unlocking the full potential of stem cells for disease understanding and treatment.
Understanding Human Pluripotent Stem Cells
Human pluripotent stem cells, capable of developing into any type of cell in the body, hold immense promise in disease modeling, drug screening, regenerative medicine, and tissue engineering. The demand for these cells is rapidly increasing in clinical and pharmaceutical settings.
Challenges in Stem Cell Production
Despite their potential, producing stem cells at the necessary scale for research and healthcare applications has been challenging due to expensive culture methods or unsafe substances unsuitable for clinical use.
Breakthrough Methodology
Published in Nature Communications in July, researchers from The University of Nottingham’s Wolfson Centre for Stem Cells, Tissue Engineering and Modelling, Uppsala University, and GE Healthcare in Sweden have developed and enhanced human stem cell culture techniques. They have identified a method using Inter-alpha inhibitor, a protein derived from human blood, to grow human pluripotent stem cells in a minimal medium without costly biological substrates.
Advantages of Inter-alpha Inhibitor
Inter-alpha inhibitor, abundant in human blood and a by-product of drug purification, promotes stem cell attachment to unmodified tissue culture plastic. This innovation eliminates the need for coating in defined human pluripotent stem cell culture and enhances cell survival in challenging conditions.
Cost and Efficiency Benefits
This breakthrough method is the first to eliminate the requirement for pre-treated biological substrates, making it more cost-effective and efficient for large-scale production. It holds promise for accelerating high-throughput cultures essential for both basic research and commercial applications.
Future Directions
Future research aims to integrate Inter-alpha inhibitor with advanced hydrogel technologies to further refine cell differentiation control and disease modeling capabilities. The focus includes rare conditions such as Multiple Osteochondroma, aiming to replicate cellular environments accurately for disease modeling.
Research Impact and Recognition
Dr. Sara Pijuan-Galitó, the study’s lead author and a Swedish Research Council Research Fellow at Nottingham, continues to advance this work under the Sir Henry Wellcome Postdoctoral Fellowship. Collaborating with Professor Morgan Alexander and Professor Chris Denning, pioneers in regenerative medicine, the team seeks to develop an economical and safe method for large-scale human stem cell culture.
The study titled “Human serum-derived protein removes the need for coating in defined human pluripotent stem cell culture” was published in Nature Communications in July 2016.
- Published in Blog
Scientists Confirm Reprogrammed Adult Stem Cells Identical to Embryonic Stem Cells
MONDAY, 19 SEPTEMBER 2016 / PUBLISHED IN BLOG
Russian researchers have concluded that reprogramming does not create differences between reprogrammed and embryonic stem cells.
Understanding Stem Cells and Reprogramming
Stem cells are specialized, undifferentiated cells with the potential to develop into various cell types in the body. Pluripotent stem cells, capable of generating any cell type, are naturally found in early embryos. They are crucial for internal repair and growth during early life.
Reprogramming Techniques and Their Importance
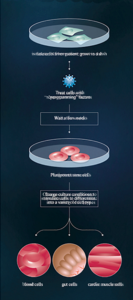
• Isolate cells from patient; grow in a dish •
Treat cells with “reprogramming”
• Wait a few weeks
• Pluripotent stem cells
• Change culture conditions to stimulate cells to differentiate into a variety of cell types
• blood cells | gut cells | cardio muscle cells
Credit: Moscow Institute of Physics and Technology
Reprogramming adult cells involves activating genes typically active in stem cells and deactivating those responsible for cell specialization. This pioneering work by Shinya Yamanaka, Nobel laureate, demonstrated that specific proteins could convert adult cells into pluripotent stem cells, known as induced pluripotent stem cells (iPSCs). This breakthrough avoids ethical concerns associated with using embryonic stem cells.
Potential Applications in Medicine
Stem cells hold promise for treating various diseases. Examples include transplanting retinal pigment epithelium and spinal cells, as well as regenerating teeth in mice. Reprogrammed iPSCs offer a revolutionary approach by allowing personalized treatment using a patient’s own cells.
Scientific Comparison: iPSCs vs. Embryonic Stem Cells
Recent studies have highlighted similarities and differences between iPSCs and embryonic stem cells. Researchers compared isogenic iPSC lines reprogrammed from different adult cell types previously derived from embryonic stem cells. Analysis of transcriptomes and methylated DNA areas showed comparable gene activity regulation mechanisms.
Research Findings and Implications
The study, published in the journal Cell Cycle, concluded that reprogramming adult cells into iPSCs does not leave distinguishing marks compared to embryonic stem cells. Differences observed were attributed to random factors rather than the reprogramming process itself.
“We defined the best induced pluripotent stem cell line concept,” says Dmitry Ischenko, Ph.D., researcher at the Moscow Institute of Physics and Technology.
Future Directions in Stem Cell Research

While this research doesn’t propose organ growth in vitro, it represents a crucial step toward understanding how specialized cells develop from pluripotent cells. Both iPSCs and embryonic stem cells offer potential for generating replacement cells and tissues to treat currently untreatable diseases.
Conclusion
The study, titled “An integrative analysis of reprogramming in human isogenic system identified a clone selection criterion,” involved researchers from the Vavilov Institute of General Genetics, Research Institute of Physical Chemical Medicine, and the Moscow Institute of Physics and Technology (MIPT).

- Published in Blog
Adult tissues that serve as sources for stem cells
MONDAY, 29 AUGUST 2016 / PUBLISHED IN BLOG
Adult stem cells are gaining significant attention in the scientific community due to their ability to self-renew and generate various types of cells and tissues. Unlike embryonic stem cells, which can differentiate into multiple cell types, adult stem cells primarily produce the specific tissue from which they originate. This focus on adult stem cells has intensified amidst ethical concerns surrounding the use of embryonic stem cells.
Diversity of Adult Stem Cell Sources
Research has identified several adult tissues that harbor stem cells, offering promising avenues for treating degenerative conditions such as osteoarthritis, muscular dystrophy, and Alzheimer’s disease. The expanding list of these tissues includes:
- Bone Marrow: Known for hematopoietic stem cells (HSCs) that can differentiate into blood cells and skeletal stem cells (STCs) contributing to bone and cartilage.
- Brain Tissue: Neural stem cells (NSCs) capable of generating various types of neurons, under study for conditions like multiple sclerosis and Parkinson’s disease.
- Peripheral Blood and Blood Vessel Tissue: Contains hematopoietic stem cells critical for immune function and blood clotting.
- Skeletal Muscle Tissue: Houses muscle stem cells important for muscle repair and regeneration.
- Liver and Pancreas Tissue: Liver’s hepatocytes possess regenerative capabilities, while pancreatic precursor cells from the biliary tree show promise in diabetes research.
Applications in Regenerative Medicine
Adult stem cells offer practical advantages over embryonic stem cells, including lower rejection rates (often sourced from the patient) and higher differentiation potential. This makes them valuable for developing therapies to treat currently incurable diseases in humans.
Future Directions in Stem Cell Research
Scientific advancements continue to uncover new insights into adult stem cells, their capabilities, and potential applications in regenerative medicine. Ongoing research aims to harness these cells’ regenerative properties more effectively, paving the way for novel treatments and therapeutic strategies.
References
For further details, refer to the original research articles and sources:

- Published in Blog
Stem cells may be used for healing damaged lungs
MONDAY, 29 AUGUST 2016 / PUBLISHED IN BLOG
Recent studies from the Weizmann Institute of Science suggest promising applications of stem cells in repairing damaged lung tissue. This breakthrough offers hope for treating prevalent conditions such as bronchitis, asthma, cystic fibrosis, and emphysema, which collectively affect millions globally and rank among the leading causes of death.
Bone Marrow Stem Cells: Potential for Lung Tissue Regeneration
Researchers at the Weizmann Institute have explored the similarities between lung and bone marrow stem cells. They found that bone marrow stem cells, when transplanted into patients, can migrate through the bloodstream to damaged lung areas and differentiate into lung tissue.
Acknowledging these similarities, Professor Yair Reisner from the Immunology Department conducted experiments where lung stem cell compartments were cleared in mice models before introducing bone marrow stem cells. This approach allowed transplanted stem cells to populate the lungs, differentiate into functional lung tissue, and significantly improve respiratory function over time.
The Weizmann team aims to expand this research to establish a bank of lung-specific stem cells for potential future transplantation in patients with severe respiratory diseases.
Lung-Specific Induced Pluripotent Stem Cells (iPSCs): A Promising Alternative
In parallel research, scientists at the Boston University Medical Center have generated lung-disease-specific induced pluripotent stem cells (iPSCs) from patients with conditions like emphysema and cystic fibrosis. These iPSCs offer advantages over bone marrow stem cells, including easier cultivation and reduced risk of rejection due to genetic similarity to the patient’s own cells.
By manipulating skin stem cells into iPSCs capable of differentiating into lung tissue, researchers have demonstrated their potential in laboratory settings. This approach could circumvent several challenges associated with other stem cell therapies, making it a viable option for future clinical applications.
References
For further reading and detailed studies:
- Published in Blog
The Language of Stem Cell Medicine: What are They? What Makes Them so Special? And What do all Those Acronyms Mean?
SUNDAY, 28 AUGUST 2016 / PUBLISHED IN BLO
Stem cell medicine leverages the body’s innate ability to heal itself, offering alternatives to conventional drugs and invasive surgeries. Stem cell therapies involve engineering human stem cells to repair or restore damaged organs and tissues, aiming to reinstate normal function.
Types of Stem Cell Therapies
Stem cell therapies encompass a range of approaches, including progenitor cells like those used in bone marrow transplants and cellular products such as platelet-rich plasma (PRP). While PRP and progenitor cells have been extensively used in clinical settings, stem cell therapies are rapidly advancing to address various medical conditions.
Autologous vs. Allogeneic Stem Cells
Stem cell treatments typically fall into two categories:
Autologous Stem Cells
Derived from the patient’s own body, autologous stem cells are used exclusively for the patient’s benefit. This approach minimizes the risk of rejection and allows for immediate treatment without the need for donor matching. Common sources include bone marrow and adipose tissue, facilitating therapies ranging from orthopedic conditions to wound healing.
Allogeneic Stem Cells
Harvested from donors, allogeneic stem cells undergo rigorous testing for diseases and are often cultured to increase cell counts. These stem cells, regulated under strict FDA guidelines, hold potential for large-scale production and widespread therapeutic applications across diverse patient populations.
Understanding Stem Cell Types
Adult Stem Cells (ASCs)
Found in various tissues, adult stem cells replenish dying cells and regenerate tissues like bone, cartilage, and adipose tissue. Over 2,000 clinical trials worldwide have explored their therapeutic potential, primarily from bone marrow and adipose tissue sources.
Induced Pluripotent Stem Cells (iPSCs)
Derived from adult cells, iPSCs are genetically reprogrammed to exhibit pluripotency, meaning they can differentiate into any cell type. Despite their versatility, iPSCs carry higher risk profiles compared to adult and embryonic stem cells.
Embryonic Stem Cells (ESCs)
Derived from human embryos, embryonic stem cells are a subject of ethical debate but remain vital for understanding regenerative processes and advancing medical research.
Types of Adult Stem Cells
Bone Marrow Stem Cells

First identified in bone marrow, these stem cells play crucial roles in healing bones and replacing blood cell types. FDA-approved under specific conditions, their use requires meticulous lab cultivation and regulatory oversight.
Adipose-Derived Stem Cells
Discovered in fat tissues, adipose-derived stem cells (ADSCs) can differentiate into diverse cell types, revolutionizing fields from plastic surgery to regenerative medicine.
- Published in Blog
Adipose Stem Cell Therapies are Revolutionizing Plastic Surgery, and Demand is High
THURSDAY, 25 AUGUST 2016 / PUBLISHED IN BLOG
Adipose stem cells (pictured) harvested from body fat. (Photo: Genetic Engineering & Biotechnology News).
Introduction to Adipose Stem Cells
The discovery of abundant stem cell populations in body fat tissue changed everything the medical community thought it knew about stem cells overnight. Now, adipose stem cell therapies are driving the plastic and cosmetic surgery industries, and demand among patients keeps rising.
Discovery of Adipose Stem Cells
In 2001, researchers and plastic surgeons from the University of Pittsburgh discovered that human fat tissue is a very rich source of mesenchymal stem cells (MSCs), multipotent stromal cells that can differentiate into a variety of cell types. When their findings were published in the Tissue Engineering Journal, the discovery stirred quite an epiphany in the medical and scientific community—until then, adult MSCs were predominantly believed to be strictly a bone marrow product. Little did those researchers realize at the time that their discovery would revolutionize cosmetic surgery in less than a decade.
Advantages of Adipose Tissue Over Bone Marrow
Adipose tissue offers distinct advantages over bone marrow tissue. Adipose fat is easier to extract than bone marrow, and the stem cell population contained in fat tissue is far more abundant than in bone marrow. One ounce of fat contains 300-500 times as many mesenchymal stem cells as an ounce of bone marrow. And unlike bone marrow, because of autologous adipose tissue’s copious stem cell count, most procedures using them do not require cells to be expanded in a lab, which means that most adipose stem cell therapies can be performed in the same operative procedure. Because bone marrow typically needs to be culture expanded for days in a lab before they can be re-injected back into a patient and adipose cells do not, there are plenty of advantages to adipose stem cell therapies.
Adipose Stem Cell Therapy Procedures
Over the past 10 years, plastic surgeons have established safe and convenient ways to remove fat and isolate the stem cells for use in cosmetic procedures. And since adipose stem cells are extracted and reintroduced to the patient’s own body, the risk of rejection that goes with donor stem cells is eliminated. Scores of ongoing clinical trials using adipose stem cells have already proven their safety and efficacy in a variety of applications. Anti-aging therapies using adipose stem cells, for instance, have grown exponentially in popularity.
Anti-Aging Benefits of Adipose Stem Cells

Cosmetic cell assisted fat transfer
As we age, cells become progressively damaged over time from sun, toxins in the environment, and the natural loss of moisture that keeps youthful skin full and wrinkle-free. Adipose stem cells work to regenerate and repair that damaged tissue, and adjunctive treatments can potentially slow down or reverse the aging process. Those cells possess a unique anti-aging effect by means of regenerating and repairing organs—including skin—damaged by environmental elements we are exposed to in our daily life, and by improving immune functions.
International Demand for Stem Cell Anti-Aging Therapies
This discovery has created an international demand for stem cell anti-aging therapies, which since these procedures are non-invasive (no surgery involved), make for a faster recovery and significantly less downtime for patients. Many patients and physicians feel that adipose stem cells also create a more natural appearance for recipients than traditional cosmetic surgery procedures. Some cosmetic stem cell physicians have taken it up a notch with cell-assisted fat transfer, in which autologous adipose-derived (stromal) stem cells are used in combination with lipoinjection for even softer, more natural results.
Procedure of Cell-Assisted Fat Transfer
Here’s how it works: a stromal vascular fraction (SVF) containing ASCs is freshly isolated from half of the aspirated fat and recombined with the other half. This process converts relatively ASC-poor aspirated fat to ASC-rich fat, reducing the potential for postoperative atrophy of injected fat to a minimal level, which clinical trials have found does not change substantially after two months.
Adipose Tissue as a Regenerative Therapy
While adipose tissue is a definitive source of stem cells, what if you don’t need to isolate or separate the stem cells to benefit from their regenerative powers?
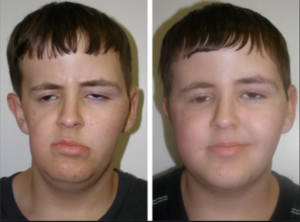
Radiation to treat a tumor severely affected this boy’s facial growth and development, caused significant facial asymmetry and left a large cosmetic defect on the side of his head. Fat grafting surgery restored his appearance to that of any healthy, happy, 14-year-old.
Regenerative Properties of Fat Grafting
Plastic surgeons have known for years that fat grafting itself, without extracting the stem cells, has regenerative properties. Cosmetic surgeons have developed safe and predictable techniques for fat grafting and have documented the regenerative effects of fat grafting in different tissues, for a variety of conditions and diseases. Adipose stem cell-rich fat grafting has been documented to reverse radiation tissue damage, something that was considered irreversible until recently. Current clinical studies are documenting the regenerative effects of fat grafting in areas no one suspected, such as autoimmune diseases and degenerative joint disease. Unlike bone marrow tissue, adipose tissue is easy to extract, it’s abundant, and it’s effective in ways researchers have only begun to discover. Cell-assisted fat grafting serves a valuable role in helping people with disfiguring injuries and birth defects.
Advancements in Adipose Tissue Applications
Plastic surgeons have acquired decades of experience in harvesting and refining adipose tissue for treating patients. Thanks to the remarkable level of expertise they have developed with adipose tissue, experts now play a leading role in developing its evolving regenerative applications. Regenerative medicine is changing the landscape of cosmetic and reconstructive surgery, and aesthetic medicine—and it keeps getting better!
- Published in Blog
Regenerating and Restoring Brain Cells in the Aged With Donor Neural Stem Cells
TUESDAY, 12 JULY 2016 / PUBLISHED IN BLOG
Introduction to Brain Plasticity and Aging
The human brain, as it turns out, is far more malleable than we once thought, even adult brains. However, they are subject to age-related diseases and disorders, such as dementia and diminished cognitive function. There is hope that medical science may be able to replace brain cells and restore memory in aging patients thanks to new discoveries in neural stem cell techniques.
New Techniques in Neural Stem Cell Research
Researchers at the Texas A&M Health Science Center College of Medicine recently published new findings in the journal Stem Cells Translational Medicine. These findings suggest a new technique for preparing donor neural stem cells and grafting them into an aged brain can regenerate tissue that has succumbed to structural, chemical, and functional changes, as well as a host of neurocognitive changes that can be attributed to aging.
Key Findings from the Study
The study, titled “Grafted Subventricular Zone Neural Stem Cells Display Robust Engraftment and Similar Differentiation Properties and Form New Neurogenic Niches in the Young and Aged Hippocampus,” was led by Ashok K. Shetty, Ph.D., a professor in the Department of Molecular and Cellular Medicine. Shetty is also the associate director of the Institute for Regenerative Medicine and a research career scientist at the Central Texas Veterans Health Care System.
Focus on the Aged Hippocampus
Shetty and his team at Texas A&M focus on the aged hippocampus, which plays an important role in making new memories and connecting them to emotions. They took healthy donor neural stem cells and implanted them into the hippocampus of an animal model, enabling them to regenerate tissue.
Significance of the Hippocampus
“We chose the hippocampus because it’s so important in learning, memory, and mood function,” Shetty said. “We’re interested in understanding aging in the brain, especially in the hippocampus, which seems particularly vulnerable to age-related changes.” The volume of this part of the brain decreases during the aging process, potentially related to a decline in neurogenesis (production of new neurons) and memory deficits.
Challenges in the Aged Hippocampus
The aged hippocampus exhibits signs of age-related degenerative changes, such as chronic low-grade inflammation and increased reactive oxygen species. Bharathi Hattiangady, assistant professor at the Texas A&M College of Medicine and co-first author of the study, was excited to discover that the aged hippocampus can accept grafted neural stem cells as well as the young hippocampus does, a discovery with significant implications for treating age-related neurodegenerative disorders.
Neural Stem Cell Grafting Process
In this new study, the team found that neural stem cells engrafted well onto the hippocampus in both young and older animal models. Not only did these implanted cells survive, but they also divided several times to create new cells. “They had at least three divisions after transplantation,” Shetty said. “The total yield of graft-derived neurons and glia was much higher than the number of implanted cells, in both the young and aged hippocampus.”
Creation of New Neural Stem Cell Niches

In both old and young brains, a small percentage of the grafted cells retained their stemness feature—an essential characteristic of a stem cell that distinguishes it from ordinary cells—and continuously produced new neurons. This process creates a new ‘niche’ of neural stem cells, which continue to produce new neurons at least three months after implantation.
Comparison to Previous Efforts
Past efforts to rejuvenate brains using fetal neurons were not as successful. Immature cells, such as neural stem cells, can tolerate the hypoxia (lack of oxygen) and trauma of the brain grafting procedure better than relatively mature neurons. The research team used a new technique of preparing the donor neural stem cells, leading to these promising results.
The Role of Brain Marrow
The researchers used donor cells from the sub-ventricular zone of the brain, an area called the “brain marrow,” analogous to bone marrow. This area holds a number of neural stem cells that persist throughout life, continuously producing new neurons that migrate to the olfactory system and respond to injury signals.
Potential of Induced Pluripotent Cells from Skin
Even a small stem cell sample can be expanded in culture, making the procedure minimally invasive. In the future, a single skin cell might suffice to create induced pluripotent stem cells, which can be pushed into neural stem cells. This eliminates the need to obtain cells from the brain, potentially revolutionizing the process.
Future Research Directions
Although the success of the grafted cells is promising, further research is needed to determine if the increased grey matter improves cognition. “Next, we want to test the impact of the implanted cells on behavior and determine if implanting neural stem cells can reverse age-related learning and memory deficits,” Shetty said. He is focused on rejuvenating the aged brain to promote successful aging, maintaining normal cognitive function, and the ability to make memories.
- Published in Blog
Stem Cell “Tattoo” Technology Allows Researchers to Track Cell Implants Non-invasively
TUESDAY, 12 JULY 2016 / PUBLISHED IN BLOG
Introduction to Stem Cell Tattoo Technology
Researchers at the University of Toronto have developed a tracer ink—a “stem cell tattoo”—that provides the ability to monitor stem cells in unprecedented detail after they’re injected. The research findings, titled “Bifunctional Magnetic Silica Nanoparticles for Highly Efficient Human Stem Cell Labeling,” were published in June in the Journal of Magnetic Resonance Imaging. Already emerging as an ideal probe for noninvasive cell tracking, the technology has the potential to revolutionize stem cell research by arming scientists with the ability to visually follow the pathways and effectiveness of stem cell therapies in the body in real time.
Advancements in Stem Cell Tracking
University of Toronto biomedical engineering professor Hai-Ling Margaret Cheng, a biomedical engineer who specializes in medical imaging, says the new technology allows researchers to actually see and track stem cells after they’re injected. Cheng hopes the technique will help expedite the development and use of stem cell therapies.
Development of the Contrast Agent
Working with colleague Xiao-an Zhang, an assistant professor of chemistry at the University of Toronto, Scarborough, Cheng developed a singular chemical compound known as a contrast agent that acts as a tracer. Composed of manganese, an element that naturally occurs in the body, this tracer compound, called MnAMP, bathes stem cells in a green solution, rendering them traceable inside the body under MRI.
Long-term Cell Tracking with Tracer Ink
The contrast agent “ink” first enters a stem cell by penetrating its membrane. Once inside, it stimulates a chemical reaction that prevents it from seeping out of the cell the same way it entered. Previous versions of contrast agents easily escaped cells. By establishing a way to contain the ink within the cell’s walls, the research team achieved the ability to track the cells long term once they are inside the body.
Advantages of the New Technology
According to Cheng, some basic contrast agents are already available for use in humans, but none are capable of tracking cells over a long period of time. Contrast agents work by illuminating the deepest and darkest corners of a person’s internal architecture so they appear clearly under X-rays, computed tomography (CT) scans, and MRIs. An example of a currently used contrasting agent would be the barium sulfate solution given to patients to help diagnose certain disorders of the esophagus, stomach, or intestines.
Non-invasive Cell Tracking Benefits
Before the stem cell tattoo tracer ink was developed, surgery was the only option for scientists to get a literal glance of a cell’s destiny after it was injected into the body. Now, researchers can track the results in real time without resorting to any invasive procedures. “Before, we could not visually track the cells once they were introduced into the body,” Cheng says. “Now we have the ability to view cells in a non-invasive manner using MRI, and monitor them for potentially a very long time.”
Current Stage of Development

Currently, the tracer ink technology is still in the early development phase and requires more animal testing. Cheng is hopeful it can proceed to human clinical trials in about 10 years. While Cheng has already proven that tattooing an animal’s embryonic stem cell doesn’t affect its ability to transform into a functional heart cell, larger models, such as rats or pigs (which better represent human size), are up for evaluation next.
Future Testing and Applications
In those test cases, researchers will cut off and reduce blood flow in the animals to mimic the effects of damage caused by a human heart attack. Cardiac stem cells pre-tagged with Cheng’s ink tracer technology will then be injected into the damaged tissue. Using MRI to monitor the luminous inked stem cells in action, researchers can non-invasively follow where in the body they’re traveling and more easily determine if the new cells are responsible for restoring normal heart rhythm. Before it can be tested in humans, the chemical tracer will also have to pass rigorous toxicology tests to ensure its safety.
- Published in Blog