Stem Cell Center Opens Facility in Argentina, Bringing Advanced Health Technology
December 24th, 2021: Stem Cell Center has opened a new location in Córdoba, Argentina. Looking to bring regenerative medicine technologies to Argentina, the grand opening of the center was attended by 80 attendees at the new IMEB facilities, and broadcast for viewers across Argentina and the rest of the world to stream online.
As a division of Global Stem Cells Group, Stem Cell Center is an international network of medical practices dedicated to bringing stem cell therapies to patients across the world. By uniting a group of medical practitioners in the field, they are able to provide training, equipment, and resources to practitioners who in turn offer research and knowledge to the community. As a group, they are continuously developing new treatments, making them available, and bringing the best possible care to patients in the pursuit of stem cell technology.
The launch of the new facility brings the group one step closer to their shared goal of reducing human suffering across the world, now making strides to provide access to these medical technologies in Argentina. Each new launch contributes to the acceleration of the process of developing and translating stem cell research into treatments and therapies that are accessible to both patients and physicians.

“Opening the center in Córdoba is a major step toward that goal, allowing us to give solutions to patients without drugs, instead manifesting the potential that lies within the body itself to trigger biological regeneration processes,” says Benito Novas, Founder of Global Stem Cells Group. This latest opening was made possible by a partnership and alliance between founder of Global Stem Cells Group, Benito Novas, and Director of IMEB, Doctor Luis Alejandro Mazzarini.
“This inauguration marks the launch of the pursuit of regenerative medicine, cell therapy, and tissue engineering treatments in Córdoba,” says Benito Novas. “I founded the group in 2010, as an expert in marketing, life sciences, and healthcare development. At the time, I was interested in the new progress that was being made in the field and was eager to make them available to as wide an array of patients as possible across the world.”
“We believe stem cell medicine is the future of the medical industry, and we work closely with regulators, policymakers, and medical professionals to foster this technology and harness the true potential.”
The seminal launch of this science, research and medical facility is one effort in the global mission of Stem Cell Center, who have announced a partnership with Bioscience Cell factory, offered training in Bolivia, and opened centers in Spain, Mexico, and Iraq. The global effort to reduce suffering is achieved one facility at a time, through the dedication of scientists, doctors, and researchers who believe in the untapped and often inaccessible potential of stem cell regenerative technology.
To learn more about the Stem Cell Center, visit their website here: https://stemcellcenter.net/about/
- Published in News
ISSCA organizes regenerative medicine symposium in alliance with IMEB in Córdoba, Argentina
The World Symposium on Stem Cells and Regenerative Medicine attracted a notable global audience interested in exploring the latest trends in regenerative medicine
MIAMI LAKES, Florida (December 22, 2021)—The International Society for Stem Cell Application (ISSCA), a multidisciplinary community of scientists and physicians collaborating to treat diseases and lessen human suffering through science, technology, and regenerative medicine, recently hosted a two-day symposium in Córdoba, Argentina. Held on December 10-11, 2021, the symposium featured onsite and virtual attendance options, attracting a large global swath of physicians, medical specialists, surgeons, dentists, veterinarians, and other professionals interested in regenerative medicine.
ISSCA hosted the event in conjunction with the support of the Argentine Society of Biological and Holistic Medicine (SAMByH) and the Global Stem Cells Group at the facilities of the Institute of Evolutionary and Biological Medicine (IMEB) in Río Cuarto. Eighty attendees were invited to attend the onsite symposium, while hundreds of additional attendees across the globe joined in virtually.
The symposium featured a number of notable experts across the world sharing their insight and expertise on various emerging topics and trends in regenerative medicine. Topics included general cell biology concepts, treatment of various diseases and conditions using regenerative medicine, uses of regenerative practices in aesthetic medicine, and more. Attendees were able to gain additional insight through question-and-answer panels with symposium presenters. Throughout the two-day event, attendees also enjoyed networking opportunities.
“The ISSCA is pleased to have collaborated with SAMByH and IMEB to bring the World Symposium on Stem Cells and Regenerative Medicine to the world. It was a resounding success, and we are thrilled that attendees took away valuable insight into the current state of and future projections for the world of regenerative medicine,” said Benito Novas, ISSCA Director. “Our goal is to help physicians and practitioners better treat their patients suffering from degenerative diseases by giving them the tools and education they need to implement regenerative protocols in their own practices. Our latest symposium has done just that, furthering the advancement of attendees’ knowledge and interest in the field as a viable treatment protocol for their patients.”
“We are thrilled to have collaborated with the ISSCA in bringing this symposium to life,” said Dr. Alejandro Mazzarini, Director of IMEB. “It was exciting to share our facility and the important work we’re doing at IMEB with the world and have such a high caliber of attendees join us for this important event.”
To learn more about the ISSCA’s latest news and innovations, visit https://www.issca.us/.
About ISSCA
The International Society for Stem Cell Application (ISSCA) is a multidisciplinary community of scientists and physicians, all of whom aspire to treat diseases and lessen human suffering through advances in science, technology, and the practice of regenerative medicine. ISSCA serves its members through advancements made to the specialty of regenerative medicine.
The ISSCA’s vision is to take a leadership position in promoting excellence and setting standards in the regenerative medicine fields of publication, research, education, training, and certification.
As a medical specialty, regenerative medicine standards and certifications are essential, which is why ISSCA offers certification training in cities all over the world. The goal is to encourage more physicians to practice regenerative medicine and make it available to benefit patients both nationally and globally. Incorporated under the Republic of Korea as a non-profit entity, the ISSCA is focused on promoting excellence and standards in the field of regenerative medicine.
- Published in News
Does Stem Cell Therapy Help With Osteoarthritis?
FRIDAY, 24 SEPTEMBER 2021 / PUBLISHED IN BLOG
Overview
Joint pain is one of the most common complaints in medicine. It is more prevalent in seniors whose joints have been subject to long-term wear and tear.
Many people develop conditions like osteoarthritis (OA) and rheumatoid arthritis (RA). The former is more common in women.
Although the pain may be temporary, it can also last for months or even years. For this reason, researchers suggested stem cell therapy to address joint disorders.
In this article, we will discuss how stem cell therapy can be useful in treating joint conditions such as osteoarthritis.
Knee Pain: What Can I Do?
Stem Cell Therapy and Arthritis
According to the Centers for Disease Control and Prevention, 54.4 million Americans (22.7%) suffered from arthritis during 2013–2015.
The number is likely to be underestimated because people don’t always seek medical attention. Instead, they prefer to use home remedies to soothe the pain.
These statistics are still extremely troubling because of the poor functional prognosis associated with arthritis.
Once you get diagnosed with arthritis, several treatments could be prescribed, including acetaminophen – it is generally the first-line treatment.
Non-Steroidal Anti-Inflammatory Drugs (NSAIDs) are the second. These include naproxen, diclofenac, and ibuprofen.
Side effects of NSAIDs include:
- Stomach ulcers
- Mineral deficiencies
- Increased bleeding risk
Corticosteroids (e.g., prednisone) are another alternative to NSAIDs. These drugs get used when NSAIDs fail to work. Side effects of drugs such as prednisone can be very serious, presenting as:
- Cushing syndrome
- High blood sugar
- Osteoporosis
- Immunosuppression
Non-pharmacological treatments such as cryotherapy or thermotherapy are the final options for treating joint problems.
How Can Cell Therapy Help With Arthritis?
Many treatment options for joint disorders can cause serious side effects, which may be more severe than the original symptoms. Therefore, scientists are always looking for alternative therapies that could help patients without causing an array of side effects.
Enter: Stem Cell Therapy
Patients with osteoarthritis often undergo joints tissue degeneration. Their articulations start to lack structural elements, including bone, cartilage, and ligaments. From this concept, the idea of using stem cell therapy to treat arthritis originated.
Because stem cells have the ability to differentiate into any cell type, they could replace the lost tissue and help restore joint function.
While this treatment may not cure all joint disorders, it is still a viable option that should be discussed. The need for stem cell therapy becomes more pronounced when your symptoms do not respond to conventional therapies.
The best part about stem cell therapy is its side effect profile. Unlike other treatments, stem cell therapy barely causes any adverse effects. In other words, it is completely safe to use on almost everyone.
Here you can check more information about Osteoarthritis and our treatments: Osteoarthritis Treatments
Takeaway Message
Many medical institutions are now incorporating stem cell therapy in their treatment plans. Orthopedic surgery is one of the most popular.
Hopefully, the use of stem cell therapy will become more mainstream to help as many patients as possible.
If you want to learn more about stem cell therapy, please do not hesitate to check out this link: Stem Cell Therapy
- Published in Blog
The Potential Benefits of Stem Cells for Concussions – What You Need To Know (2021)
THURSDAY, 23 SEPTEMBER 2021 / PUBLISHED IN BLOG
Overview
Traumatic brain injuries (TBI), also known as concussions, are among the most frequent causes of death in the United States.
According to the Centers for Disease Control and Prevention (CDC), the number of emergency department visits related to TBI admissions, complications, and deaths increased by 53% in recent years.
People who can recover from TBI may experience irreversible neurological issues throughout their lives. As a result, they lose their autonomy and become reliant on other people to perform daily activities.
However, it’s not all doom and gloom. A few studies suggest that stem cell therapy could be beneficial in the management of TBI.
In this article, we’ll discuss everything you need to know about the potential effects of stem cell therapy in managing concussions.
How Can Stem Cells Help With Concussions?
For those unfamiliar with how stem cells function, here is a short description:
Stem cells can develop into any kind of tissue or cell, including those that don’t typically regenerate (e.g., heart cells, nerve cells). For instance, medical schools often teach that a person has the same number of neurons from the moment they are born to their death.
Although this assertion is not completely true due to the latest evidence that supports the ability of neurons to regenerate, the rate of regeneration is nowhere near enough to restore the damaged tissues caused by TBI or strokes.
To this end, researchers are using stem cells to create new neurons to help patients with TBI, multiple sclerosis, and other neurological disorders.
What is the Scientific Consensus on Stem Cell Therapy and Traumatic Brain Injuries?
Mesenchymal stem cells derived from the bone marrow, umbilical cord, or adipose tissues showed positive results in the management of concussions.
In a number of studies, the administration of stem cell therapy via the spinal cord showed real evidence of lesion improvement. Researchers used advanced imaging techniques such as functional MRI to objectify the changes.
Neuroscientists theorize that stem cells can replace the dead neurons, which helps with restoring some of the lost functions.
Today, hundreds of clinical trials are trying to unveil the full extent of how stem cell therapy helps with traumatic brain injuries.
Based on the available research, scientists advocate for the use of stem cell therapy in treating patients with severe concussions.
However, neuroscientists insist that stem cell therapy is only used in patients who failed to respond to conventional treatment methods.
Are You a Candidate for Stem Cell Therapy?
To see whether you are a candidate for stem cell therapy, contact us by clicking on this link. You will speak with one of our healthcare professionals in Cancun, México.

Takeaway Message
Stem cells have an amazing ability to divide into any type of tissue and potentially restore lost functions.
We hope that this article managed to highlight the potential role of stem cell therapy in addressing traumatic brain injury.
If you have any questions or concerns regarding stem cell therapy or TBI, please do not hesitate to share your thoughts in the comment section below.
- Published in Blog
Can Stem Cell Therapy Help With Alopecia (hair loss)?
THURSDAY, 23 SEPTEMBER 2021 / PUBLISHED IN BLOG
Overview
Despite the impressive advancements made in the field of medicine, hair loss remains unsolved today.
Every year, hundreds of clinical trials attempt to come up with a revolutionary treatment that will restore hair loss. Unfortunately, most of these studies fail to produce sufficient results.
Interestingly, the issue with hair loss is not financial. In other words, the scientific community spent billions of dollars to find a solution for this problem. This is not the case for other dermatological conditions (e.g., rosacea).
In this article, we will briefly cover the downsides of hair loss, then switch gears to the potential effects of stem cell therapy in addressing this issue.
The Downsides of Hair Loss
While unaffected people see hair loss as a mere esthetic issue, patients with this condition experience many adverse effects, including mental health problems, such as depression, low self-esteem, and a high sense of self-consciousness.
These issues could negatively impact the quality of life of these patients.
Moreover, alopecia affects both genders in great numbers, making this a real public health issue. We should note that some cases of hair loss have underlying conditions, such as hypothyroidism, autoimmune diseases, and infections.
Stem Cell Therapy and Hair Loss
In recent years, the clinical uses of stem cell therapy have expanded, covering many fields in medicine, such as hair loss.
The primary issue with treating chronic illnesses is their irreversible nature. In other words, once the tissue is lost, there is no effective medication that can recover it.
For instance, when the cells responsible for producing hair follicles die, no drug will be able to restore them.
This is the core issue with alopecia.
Fortunately, biological treatments such as stem cell therapy and exosome therapy might just be the solution to this issue. Due to the incredible ability of stem cells to differentiate into any cell type, they could replace the cells responsible for producing hair follicles.
Typically, your doctor will extract stem cells from your own bone marrow or adipocytes (i.e., fat cells) to prevent any immunological reactions.
Using all of this information, some clinics started offering stem cell therapy to manage hair loss, using noninvasive procedures.
Usually, your doctor will schedule several sessions in order for the results to become apparent. However, many patients report positive findings after the very first therapeutic session.
The Potential of Stem Cell Therapy in Dermatology
Despite the underexploited nature of stem cell therapy, it may potentially help with numerous medical fields, including dermatology and esthetic medicine.
Takeaway Message
Hair loss is a real issue for people from all across the world. Treating this disorder is really challenging, especially when the underlying condition is unclear. Fortunately, stem cell therapy may play an important role in addressing this issue since it’s one of the few therapeutic modalities that actually restore lost tissue.
We hope that this article helped you appreciate the role of stem cell therapy in the management of alopecia.
If you want to become a specialist in stem cell therapies and help your patients, check our next training dates here: Exosomas + PRP The healing combo – Cellular Hope Institute – Cancún, México
- Published in Blog
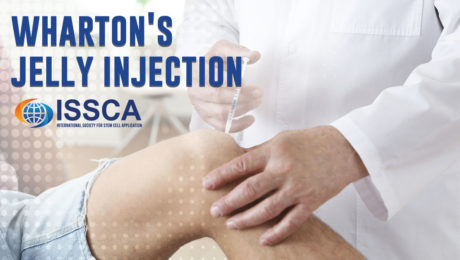
Wharton’s Jelly injection
MONDAY, 20 SEPTEMBER 2021 / PUBLISHED IN BLOG
What is Wharton’s Jelly?
Wharton’s Jelly is the gelatinous connective tissue found in the umbilical cord. This once discarded substance, previously thought of as afterbirth waste, is rich in healing substances and abundant in mesenchymal stem cells (MSCs).
Benefits of Mesenchymal Stem Cells from Wharton’s Jelly
The mesenchymal stem cells in Wharton’s Jelly are special because they are not readily recognized by the body’s immune system. They are considered “primitive cells,” meaning they have properties similar to embryonic cells. This reduces the risk of an immune reaction when these cells are injected into a patient.
Minimally processed Wharton’s Jelly preserves the growth factors and proteins needed for effective healing.
Who Can Benefit from Wharton’s Jelly Treatment?
Wharton’s Jelly is used in regenerative medicine, which employs naturally occurring cells and substances to promote tissue regeneration and healthy cell growth. This natural substance contains collagen, anti-inflammatory properties, growth proteins, and hyaluronic acid.
Treatment with Wharton’s Jelly can help with various ailments, including:
- Degenerative diseases
- Osteoarthritis
- Ligament and muscle damage
- Joint pain and inflammation
- Chronic pain
- Wound healing
There are no age restrictions on who can receive this treatment, and often a patient will only need treatment once.
How Does Wharton’s Jelly Treatment Work?
A specialized, minimally processed product derived from Wharton’s Jelly is injected into the injured site. While numbing is not always needed, lidocaine may be used for particularly sensitive areas. This process involves a relatively painless pinch of a needle. Once injected, the mesenchymal stem cells begin to transform into the cells needed to regenerate damaged tissue, reducing inflammation and pain, and restoring functionality.
Are There Any Side Effects to the Treatment?
No long-term risks or side effects have been reported. It is important to discuss proper protocols with your doctor before treatment.
Reasons to Undergo Cellular Therapy in Cancun, Mexico with Us
One of the main advantages of receiving cellular therapy treatment in Mexico is the ability to cultivate and reproduce, in a laboratory environment, the cells extracted from patients. This means a patient can receive a much larger concentration of cells, leading to improved results.
Additionally, Mexico is a great option for treatments that require a more invasive approach—a larger operating team, more medical products, and PPE. As one of the Western Hemisphere’s largest medical tourism capitals, treatments in Mexico are performed in hospitals that comply with FDA regulations and are offered at a more affordable price point.
While these treatments require a large investment from the patient, the cost of a multiple-day cellular therapy treatment in Mexico is far more affordable than the same treatment in the United States and Canada, especially without the ability to use culture-expanded cells.
What is the Recovery Period?
The actual injection does not require a recovery period, and most patients can return to their normal daily activities immediately. In cases of injury to ligaments or joints, rest may be required. Please follow your doctor’s advice for the best results from treatment.
Overall, Wharton’s Jelly injection treatment is minimally invasive and can help with a variety of ailments. With its limited side effects and regenerative properties, it is safe for patients of any age.

Contact Us
Talk with us to see if Wharton’s Jelly injection therapy is the right fit for you: Click here
About Cellular Hope Institute
At Cellular Hope Institute, we evaluate, diagnose, and treat patients around the world on a daily basis with the latest regenerative medicine modalities available today.
- Published in Blog
Wharton’s Jelly – how does it work?
THURSDAY, 16 SEPTEMBER 2021 / PUBLISHED IN BLOG
What is Wharton’s Jelly?
Wharton’s Jelly is the substance that helps lubricate and support the umbilical cord. What makes Wharton’s Jelly so special is it contains high levels of mesenchymal stem cells. These special stem cells adapt to fit the cells needed to help regenerate damaged tissue and relieve pain naturally.
The Potency of Mesenchymal Stem Cells in Wharton’s Jelly
The mesenchymal stem cells that come from Wharton’s Jelly are among the most potent stem cells, rich in regenerative properties. Wharton’s Jelly also contains a good amount of collagen, hyaluronic acid, and anti-inflammatory properties, making them quintessential in regenerative medicine.
The Role of Regenerative Medicine
Regenerative medicine refers to treatments and medicine that are naturally occurring, including stem cell therapies. Mesenchymal stem cells are harvested from the Wharton Jelly found in the umbilical cord, donated by a healthy mother of a full-term baby. These stem cells contain all the regenerative properties needed to help the body heal and relieve pain naturally.
How Does Wharton’s Jelly Treatment Work?
Mesenchymal stem cells are injected into the body and have the ability to transmute into cells needed to repair damaged tissue and relieve inflammation. These cells harness potent regenerative properties that help rebuild the injured part of the body and provide long-lasting natural pain relief. Unlike pain medicines, which provide temporary relief that masks the symptoms and can be harmful if taken long-term, the stem cells found in Wharton’s Jelly are naturally occurring and natural to the body.
Best Uses for Wharton’s Jelly
Wharton’s Jelly is best used in patients with degenerative diseases and musculoskeletal injuries. The anti-inflammatory and regenerative properties work to repair damaged tissues, leading to lasting pain relief and restored function.
What to Expect from Wharton’s Jelly Treatment
Once a patient and doctor have decided on this type of stem cell treatment, patients can expect a relatively painless process. Stem cells are injected into the site and immediately go to work. Injections are virtually painless, and there are no known negative side effects. Patients can generally get right back to the activities they love. Doctors will discuss treatment plans based on individual needs, and in some cases, resting an injured area for a specific period may be needed.
The Effectiveness of Wharton’s Jelly Therapy
The mesenchymal stem cells found in Wharton’s Jelly have proven to be a very successful therapeutic method to treat several degenerative issues and injuries. This therapy is relatively painless, with no known negative side effects, making it an excellent choice in treatment. Instead of masking the pain with dangerous medicine, stem cell therapy offers a naturally occurring solution that aims to repair the damaged tissue and resolve the problem.
Learn More About Wharton’s Jelly Treatment
If you want to learn more about Wharton’s Jelly Treatment and how you can help your patients, you can check our next training course here: Wharton’s Jelly Training Course.
- Published in Blog
ISSCA to Launch Regenerative Medicine Studies Program in Collaboration with Medicel Chile
International Society for Stem Cell Application ISSCA to Launch Postgraduate Studies Program in Stem Cell Therapies and Regenerative Medicine in Collaboration with Medicel Chile
ISSCA and Medicel, Inc. have announced plans to launch a post-graduate studies program in stem cell and regenerative medicine, to be conducted in Santiago Chile February 2021.
MIAMI, July 20 , 2020— The international Society for Stem Cell Application ISSCA and Medicel Chile have announced plans to launch a post graduate studies program in stem cell therapies and regenerative medicine in 2020.
The program will include Seven days of intensive, interactive training coursework with classroom instruction and laboratory practice through didactic lectures, hands-on practical experience in laboratory protocols and relevant lessons in regulatory practices. Medicel’s Chief Scientific Officer and Other leading Scientists will teach the coursework and perform laboratory instruction, accompanied by a series of guest lecturers from the Global Stem Cells Group faculty of scientists.
Attendees will receive hands-on training in techniques for a variety of laboratory processes, and gain insight into the inner workings of a cGMP laboratory and registered tissue bank. Regenerative medicine experts with more 15 years of experience in the field will train attendees and provide the necessary tools to implement regulatory and clinical guidelines in a cGMP laboratory setting
The graduate course will be scheduled 3 times during 2020 starting February 23rd
“ Our end goal in Launching this Fellowship program is to help physicians that are looking for really advanced and formal training in Cellular Therapies and Regenerative Medicine — We want to provide them the necessary skills and in depth specialization that is lacking in our smaller point of care programs ,” noted Benito Novas ISSCA Public Relations Director
To learn more about the February 2020 certification event in Santiago, or any of the other upcoming certification courses around the world, visit the ISSCA website.
About the International Society for Stem Cells Applications (ISSCA)
The International Society for Stem Cells Applications (ISSCA) is a multidisciplinary community of scientists and physicians, all of whom aspire to treat diseases and lessen human suffering through advances in science, technology, and the practice of regenerative medicine.
Incorporated and Trademarked in the United States of America as a non-profit entity, ISSCA is focused on promoting excellence and standards in the field of regenerative medicine.
ISSCA bridges the gaps between scientists and practitioners in Regenerative Medicine.
Their code of ethics emphasizes principles of morals and ethical conducts. ISSCA’s vision is to take a leadership position in promoting excellence and setting standards in the regenerative medicine fields of publication, research, education, training, and certification. ISSCA serves its members through advancements made to the specialty of regenerative medicine. They aim to encourage more physicians to practice regenerative medicine and make it available to benefit patients both nationally and globally.
For more information, please visit the ISSCA’s website or send an email to info@stemcellsgroup.com www.stemcellsgroup.com
About Medicel Chile
Medicel Chile is one of the premiere regenerative medicine treatment centers in the country of Chile, conveniently located in Santiago de Chile, one of the largest metropolitan areas in Latin America. Medicel carries with it a reputation as one of the most scientifically advanced and professional regenerative medicine Laboratories in Chile, and this is in no small part due to its highly-qualified team of scientists and medical professionals and advisers, who research tirelessly to head new breakthroughs in the fields of cellular therapy and cryopreservation, which is the freezing of a stem cell sample to ensure its longevity and freshness, should the patient receiving the service ever wish to use their frozen cells for some future malady.
- Published in News
Stem Cell Center Network in Lisbon Portugal
The Stem Cell Center Network ( a Division of Global Stem Cells Group ) is an international network of regenerative medicine practitioners . Dedicated to promoting the research and development of the field of regenerative medicine, and strives to bring patients cutting-edge treatments that make use of the ever-growing list of benefits that regenerative medicine carry. This week, in a part of their effort to expand their foothold in medical communities around the world, the Stem Cell Center Network has announced the opening of a new training facility in Lisbon, Portugal.
Over the years, the Stem Cell Center Network has sought out to aggressively expand and roll out new membership opportunities, programs, events, and more– an effort that has been redoubled in the last five years. As a result, we have added members that practice regenerative medicine under our banner in over twenty five countries spread across five continents.
The new Portuguese center will be located in the clinic of Dr. Hugo Madeiras, a highly-accredited physician and scientist who will preside over the facility as the Global Stem Cells Group chief representative in Portugal. As the CEO and founder of the Clinic of Advanced Implantology, he focuses exclusively on implantology, esthetics, and digital workflows, and collaborates with over twenty doctors and seventy resources in total. He is also the clinical collaborator for the Straumann Group, a faculty which includes product testing, lecturing, and the creation and delivery of educational content.
Dr. Madeiras graduated from the Instituto Superior de Ciências da Saúde Egas Moniz in Medicine in 2007, and with a Master in Oral Rehabilitation from CESPU in 2009. With his over ten year of experience in the field, he acts as a speaker at several national and international congresses that go over developments and new treatment protocols in the field of dentistry.
“This new center will help in bringing regenerative medicine to the people of Lisbon. It will treat patients with a wide variety of different diseases, but with a special emphasis on Aesthetic Medicine,” Said Benito Novas CEO of the Global Stem Cells Group about the new Stem Cell Center Network partnership, “This new facility will be at the top of the line, a place where Portuguese doctors can come to learn about regenerative medicine protocols, and the advancements that have been made in the field. I am incredibly excited to be working in partnership with Global Stem Cells Group , and look forward to many years as a program leader here in Portugal,”
Stem Cell Center Network plans for the clinic to open in September 2020– and with that date looming closer and closer with each passing week, a date has also been agreed upon for the clinic’s inaugural training– indeed, it will be a place for doctors around the country to convene and learn, share, and research the complexities and applications of regenerative medicine. Barring any extenuating circumstances, the next first Regenerative Medicine Certification Training in the Portuguese clinic will take place on September 25 & 26th, 2020– just weeks after its formal opening. With only 10 spots available, applicants are encouraged to sign up soon for the hands-on training course.
To sign up today, visit: https://issca.us
About Global Stem Cells Group
Global Stem Cells Group (GSCG) is a worldwide network that combines seven major medical corporations, each focused on furthering scientific and technological advancements to lead cutting-edge stem cell development, treatments, and training. The united efforts of GSCG’s affiliate companies provide medical practitioners with a one-stop hub for stem cell solutions that adhere to the highest medical standards.
Global Stem Cells Group is a publicly traded company operating under the symbol MSSV.
https://finance.yahoo.com/quote/mssv/
- Published in News
Global Stem Cells Group has Announced an Agreement with Rokit Healthcare
Global Stem Cells Group announces an agreement with the South Korean biotechnology giant known as Rokit Healthcare to represent the company’s technology in the Latin American market.
The Global Stem Cells Group (GSCG) a world leader in Regenerative Medicine Technologies has signed an agreement with South Korean-based Rokit Healthcare, an esteemed bioprinter manufacturer that is committed to advancing the field of regenerative medicine and bettering the quality of life of people around the world.
The field of bioprinting is an extremely new one, but it shows great promise. Simply, it is the automated, computer aided deposition of bio-materials (which are cells, growth factors, and biocompatible polymers) for the manufacturing of functional human tissues or organs. Growth factors are harvested and used with a proprietary printing technology to create or regenerative damaged or diseased organs. Rokit Healthcare does this primarily through the proliferation of a machine that they dub an ‘organ regenerator’– it looks like a 3D printer, but instead of using plastics to create things, they use cells and materials that will be safe to implant within the human body.
The process of 3D bioprinting human tissues and organs is a revolutionary technology in the field of tissue engineering. One of the major challenges in regenerative medicine research and tissue engineering is mimicking the micro and macro environment of human tissues. In response to this challenge, advances in additive manufacturing have inspired scientists in Korea to develop novel bioprinting technology, for human tissues and organs
With the advancements of 3D printing and regenerative medicine working together, the potential is seemingly limitless for the spreading of bioprinting technology, a process that is known as 4D Printing– and Global Stem Cells Group, in an effort make this revolutionary technology available to patients, has forged an agreement with Rokit Healthcare to promote, and distribute the company’s technology in Latin America-
The Invivo 4D Printer is Rokit Healthcare’s flagship product, and it is one that revolutionizes the application of regenerative medicine and growth factor-based therapies. creating a solution for personalized and improved patient care. By leveraging a combination of 3D and bioprinting technologies, it can better distribute a patient’s autologous tissues and cells, making it an invaluable tool for those that are looking to improve the efficacy of their results, especially for certain dermatological conditions including scarring.
“We’re extremely excited about this new opportunity and look forward to working with Rokit,” Says Benito Novas, CEO of the Global Stem Cells Group, “The Invivo 4D Printer is in a position to turn the practice of regenerative medicine onto its head, and we are planning on creating a training center in Cancun, Mexico exclusively to showcase and instruct other physicians in this cutting-edge technology,”
About Global Stem Cells Group
Global Stem Cells Group (GSCG) is a worldwide network that combines seven major medical corporations, each focused on furthering scientific and technological advancements to lead cutting-edge stem cell development, treatments, and training. The united efforts of GSCG’s affiliate companies provide medical practitioners with a one-stop hub for regenerative medicine solutions that adhere to the highest medical standards.
Global Stem Cells Group is a publicly traded company operating under the symbol MSSV.
https://finance.yahoo.com/quote/mssv/
About ROKIT :
ROKIT Healthcare is a global healthcare company that is committed to providing an effective and autologous organ regeneration platform. In order to undertake this daunting task, the company uses proprietary biofabrication technologies that show promise in treating several types of diseases in the field of regenerative medicine. Through the proliferation of 4D bioprinting technology, autologous stem cell technologies, ROKIT Healthcare believes that supplying an avenue for organ regeneration will drastically change the way that everyday people trust and manage their own body.
- Published in News